Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015-2017: a cross-sectional study
- PMID: 32532786
- PMCID: PMC7295430
- DOI: 10.1136/bmjopen-2020-038863
Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015-2017: a cross-sectional study
Abstract
Objectives: Little is routinely disclosed about the costs of the pivotal clinical trials that provide the key scientific evidence of the treatment benefits of new therapeutic agents. We expand our earlier research to examine why the estimated costs may vary 100-fold.
Design: A cross-sectional study of the estimated costs of the pivotal clinical trials supporting the approval of 101 new therapeutic agents approved by the US Food and Drug Administration from 2015 to 2017.
Methods: We licensed a software tool used by the pharmaceutical industry to estimate the likely costs of clinical trials to be conducted by contract research organisations. For each trial we collected 52 study characteristics. Linear regression was used to assess the most important factors affecting costs.
Primary and secondary outcome measures: The mean and 95% CI of 225 pivotal clinical trials using varying assumptions. We also assessed median estimated costs per patient, per clinic visit and per drug.
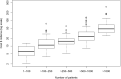
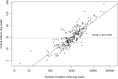
Results: Measured as pivotal trials cost per approved drug, the 101 new molecular entities had an estimated median cost of US$48 million (IQR US$20 million-US$102 million). The 225 individual clinical trials had a median estimate of US$19 million (IQR US$12 million-US$33 million) per trial and US$41 413 (IQR, US$29 894-US$75 047) per patient. The largest single factor driving cost was the number of patients required to establish the treatment effects and varied from 4 patients to 8442. Next was the number of trial clinic visits, which ranged from 2 to 166. Our statistical model showed trial costs rose exponentially with these two variables (R2=0.696, F=257.9, p<0.01).
Conclusions: The estimated costs are modest for measuring the benefits of new therapeutic agents but rise exponentially as more patients and clinic visits are required to establish a drug effect.
Keywords: clinical pharmacology; clinical trials; epidemiology; medical law; public health.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GCA is past Chair of FDA’s Peripheral and Central Nervous System Advisory Committee; serves as a paid advisor to IQVIA; is a paid consultant and holds equity in Monument Analytics, a healthcare consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation and is a member of OptumRx’s National P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Figures
References
-
- Food and Drug Administration Food and Drug Administration Amendments Act (FDAAA) of 2007, 2007. Available: https://www.fda.gov/RegulatoryInformation/LawsEnforcedbyFDA/SignificantA... [Accessed 16 Apr 2018].
-
- International Committee of Medical Journal Editors Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. Available: http://www.icmje.org/ - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical