Association between COPD exacerbations and lung function decline during maintenance therapy
- PMID: 32532852
- PMCID: PMC7476283
- DOI: 10.1136/thoraxjnl-2019-214457
Association between COPD exacerbations and lung function decline during maintenance therapy
Abstract
Background: Little is known about the impact of exacerbations on COPD progression or whether inhaled corticosteroid (ICS) use and blood eosinophil count (BEC) affect progression. We aimed to assess this in a prospective observational study.
Methods: The study population included patients with mild to moderate COPD, aged ≥35 years, with a smoking history, who were followed up for ≥3 years from first to last spirometry recording using two large UK electronic medical record databases: Clinical Practice Research Datalink (CPRD) and Optimum Patient Care Research Database (OPCRD). Multilevel mixed-effects linear regression models were used to determine the relationship between annual exacerbation rate following initiation of therapy (ICS vs non-ICS) and FEV1 decline. Effect modification by blood eosinophils was studied through interaction terms.
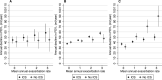
Results: Of 12178 patients included (mean age 66 years; 48% female), 8981 (74%) received ICS. In patients with BEC ≥350 cells/µL not on ICS, each exacerbation was associated with subsequent acceleration of FEV1 decline of 19.4 mL/year (95% CI 12.0 to 26.7, p<0.0001). This excess decline was reduced by 15.1 mL/year (6.6 to 23.6) to 4.3 mL/year (1.9 to 6.7, p<0.0001) in those with BEC ≥350 cells/µL treated with ICS.
Conclusion: Exacerbations are associated with a more rapid loss of lung function among COPD patients with elevated blood eosinophils, defined as ≥350 cells/µL, not treated with ICS. More aggressive prevention of exacerbations using ICS in such patients may prevent excess loss of lung function.
Keywords: COPD exacerbations; COPD pharmacology; eosinophil biology; lung physiology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MK, JV, VC and JWHk are employees of the Observational and Pragmatic Research Institute, which conducted this study and which has conducted paid research in respiratory disease on behalf of the following other organisations in the past 5 years: Aerocrine, AKL Research and Development Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Orion, Takeda, Teva, Zentiva (a Sanofi company). DBP has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Mundipharma, Napp, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Mylan, Mundipharma, Napp, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service, Zentiva (Sanofi Generics); payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Merck, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Skyepharma, Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis, Teva Pharmaceuticals; funding for patient enrolment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, Zentiva (Sanofi Generics); stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, and Health Technology Assessment. CC, PD and PD are employees of AstraZeneca. DDS has received honoraria for speaking engagements from Boehringer Ingelheim (BI), AstraZeneca (AZ) and Novartis. He has received research funding from Merck, BI and AZ for work related to COPD and has sat on advisory boards of AZ, BI and Sanofi. MS has received honoraria for speaking engagements from Boehringer Ingelheim, AstraZeneca and GlaxoSmithKline.
Figures



Comment in
-
Using routine health data for research: the devil is in the detail.Thorax. 2020 Sep;75(9):714-715. doi: 10.1136/thoraxjnl-2020-214821. Epub 2020 Jun 24. Thorax. 2020. PMID: 32580992 No abstract available.
References
-
- Global Initiative for Chronic Obstructive Lung Disease Pocket guide to COPD diagnosis, management, and prevention – a guide for health care professionals, 2016.
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) From the global strategy for the diagnosis, management and prevention of COPD, 2017. Available: https://goldcopd.org
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
