Mutations in virus-derived small RNAs
- PMID: 32533016
- PMCID: PMC7293216
- DOI: 10.1038/s41598-020-66374-2
Mutations in virus-derived small RNAs
Abstract
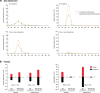
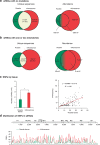
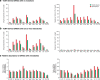
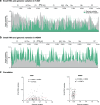
RNA viruses exist as populations of genome variants. Virus-infected plants accumulate 21-24 nucleotide small interfering RNAs (siRNAs) derived from viral RNA (virus-derived siRNAs) through gene silencing. This paper describes the profile of mutations in virus-derived siRNAs for three members of the family Potyviridae: Turnip mosaic virus (TuMV), Papaya ringspot virus (PRSV) and Wheat streak mosaic virus (WSMV). For TuMV in Arabidopsis thaliana, profiles were obtained for mechanically inoculated rosette leaves and systemically infected cauline leaves and inflorescence. Results are consistent with selection pressure on the viral genome imposed by local and systemic movement. By genetically removing gene silencing in the plant and silencing suppression in the virus, our results showed that antiviral gene silencing imposes selection in viral populations. Mutations in siRNAs derived from a PRSV coat protein transgene in the absence of virus replication showed the contribution of cellular RNA-dependent RNA polymerases to the generation of mutations in virus-derived siRNAs. Collectively, results are consistent with two sources of mutations in virus-derived siRNAs: viral RNA-dependent RNA polymerases responsible for virus replication and cellular RNA-dependent RNA polymerases responsible for gene silencing amplification.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

