A decade of advances in transposon-insertion sequencing
- PMID: 32533119
- PMCID: PMC7291929
- DOI: 10.1038/s41576-020-0244-x
A decade of advances in transposon-insertion sequencing
Abstract
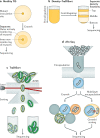
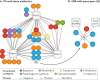
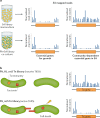
It has been 10 years since the introduction of modern transposon-insertion sequencing (TIS) methods, which combine genome-wide transposon mutagenesis with high-throughput sequencing to estimate the fitness contribution or essentiality of each genetic component in a bacterial genome. Four TIS variations were published in 2009: transposon sequencing (Tn-Seq), transposon-directed insertion site sequencing (TraDIS), insertion sequencing (INSeq) and high-throughput insertion tracking by deep sequencing (HITS). TIS has since become an important tool for molecular microbiologists, being one of the few genome-wide techniques that directly links phenotype to genotype and ultimately can assign gene function. In this Review, we discuss the recent applications of TIS to answer overarching biological questions. We explore emerging and multidisciplinary methods that build on TIS, with an eye towards future applications.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

