Noninvasive Fetal Genotyping by Droplet Digital PCR to Identify Maternally Inherited Monogenic Diabetes Variants
- PMID: 32533152
- PMCID: PMC7611030
- DOI: 10.1093/clinchem/hvaa104
Noninvasive Fetal Genotyping by Droplet Digital PCR to Identify Maternally Inherited Monogenic Diabetes Variants
Abstract
Background: Babies of women with heterozygous pathogenic glucokinase (GCK) variants causing mild fasting hyperglycemia are at risk of macrosomia if they do not inherit the variant. Conversely, babies who inherit a pathogenic hepatocyte nuclear factor 4α (HNF4A) diabetes variant are at increased risk of high birth weight. Noninvasive fetal genotyping for maternal pathogenic variants would inform pregnancy management.
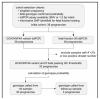
Methods: Droplet digital PCR was used to quantify reference and variant alleles in cell-free DNA extracted from blood from 38 pregnant women heterozygous for a GCK or HNF4A variant and to determine fetal fraction by measurement of informative maternal and paternal variants. Droplet numbers positive for the reference/alternate allele together with the fetal fraction were used in a Bayesian analysis to derive probability for the fetal genotype. The babies' genotypes were ascertained postnatally by Sanger sequencing.
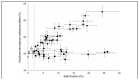
Results: Droplet digital PCR assays for GCK or HNF4A variants were validated for testing in all 38 pregnancies. Fetal fraction of ≥2% was demonstrated in at least 1 cell-free DNA sample from 33 pregnancies. A threshold of ≥0.95 for calling homozygous reference genotypes and ≤0.05 for heterozygous fetal genotypes allowed correct genotype calls for all 33 pregnancies with no false-positive results. In 30 of 33 pregnancies, a result was obtained from a single blood sample.
Conclusions: This assay can be used to identify pregnancies at risk of macrosomia due to maternal monogenic diabetes variants.
Keywords: Bayesian analysis; droplet digital PCR; monogenic diabetes; non-invasive prenatal diagnosis.
© American Association for Clinical Chemistry 2020.
Conflict of interest statement
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/orpotential conflicts of interest:
Figures

Similar articles
-
Sequencing Cell-free Fetal DNA in Pregnant Women With GCK-MODY.J Clin Endocrinol Metab. 2021 Aug 18;106(9):2678-2689. doi: 10.1210/clinem/dgab265. J Clin Endocrinol Metab. 2021. PMID: 34406393 Free PMC article.
-
Pregnancy in Women With Monogenic Diabetes due to Pathogenic Variants of the Glucokinase Gene: Lessons and Challenges.Front Endocrinol (Lausanne). 2022 Jan 5;12:802423. doi: 10.3389/fendo.2021.802423. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35069449 Free PMC article. Review.
-
Fetal Genotyping in Maternal Blood by Digital PCR: Towards NIPD of Monogenic Disorders Independently of Parental Origin.PLoS One. 2016 Apr 14;11(4):e0153258. doi: 10.1371/journal.pone.0153258. eCollection 2016. PLoS One. 2016. PMID: 27078875 Free PMC article.
-
Antenatal diagnosis of fetal genotype determines if maternal hyperglycemia due to a glucokinase mutation requires treatment.Diabetes Care. 2012 Sep;35(9):1832-4. doi: 10.2337/dc12-0151. Epub 2012 Jul 6. Diabetes Care. 2012. PMID: 22773699 Free PMC article.
-
Noninvasive Fetal RhD Blood Group Genotyping: A Health Technology Assessment.Ont Health Technol Assess Ser. 2020 Nov 2;20(15):1-160. eCollection 2020. Ont Health Technol Assess Ser. 2020. PMID: 33240456 Free PMC article. Review.
Cited by
-
Enhancing fetal outcomes in GCK-MODY pregnancies: a precision medicine approach via non-invasive prenatal GCK mutation detection.Front Med (Lausanne). 2024 Apr 30;11:1347290. doi: 10.3389/fmed.2024.1347290. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38745742 Free PMC article.
-
Clinical Diagnostic Performance of Droplet Digital PCR for Suspected Bloodstream Infections.Microbiol Spectr. 2023 Feb 14;11(1):e0137822. doi: 10.1128/spectrum.01378-22. Epub 2023 Jan 5. Microbiol Spectr. 2023. PMID: 36602351 Free PMC article.
-
Pregnancy and neonatal outcomes in women with GCK-MODY: an observational study based on standardised insulin modalities.Diabetologia. 2025 May;68(5):981-992. doi: 10.1007/s00125-025-06363-0. Epub 2025 Feb 19. Diabetologia. 2025. PMID: 39971752
-
Digital PCR: from early developments to its future application in clinics.Lab Chip. 2025 Aug 5;25(16):3921-3961. doi: 10.1039/d5lc00055f. Lab Chip. 2025. PMID: 40686367 Free PMC article. Review.
-
Non-invasive prenatal diagnosis (NIPD): how analysis of cell-free DNA in maternal plasma has changed prenatal diagnosis for monogenic disorders.Clin Sci (Lond). 2022 Nov 30;136(22):1615-1629. doi: 10.1042/CS20210380. Clin Sci (Lond). 2022. PMID: 36383187 Free PMC article.
References
-
- Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence offetal DNA in maternal plasma and serum. Lancet. 1997;350:485–7. - PubMed
-
- Boon EM, Faas BH. Benefits and limitations of whole genome versus targeted approaches for noninvasive prenatal testing for fetal aneuploidies. Prenat Diagn. 2013;33:563–8. - PubMed
-
- Hyett JA, Gardener G, Stojilkovic-Mikic T, Finning KM, Martin PG, Rodeck CH, Chitty LS. Reduction in diagnostic and therapeutic interventions by non-invasive determination of fetal sex in early pregnancy. Prenat Diagn. 2005;25:1111–6. - PubMed
-
- Clausen FB. Integration of noninvasive prenatal prediction of fetal blood group into clinical prenatal care. Prenat Diagn. 2014;34:409–15. - PubMed
-
- Chitty LS, Griffin DR, Meaney C, Barrett A, Khalil A, Pajkrt E, Cole TJ. New aids for the non-invasive prenatal diagnosis of achondroplasia: dysmorphic features, charts of fetal size and molecular confirmation using cell-free fetal DNA in maternal plasma. Ultrasound Obstet Gynecol. 2011;37:283–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

