Acute kidney injury in critically ill patients with COVID-19
- PMID: 32533197
- PMCID: PMC7290076
- DOI: 10.1007/s00134-020-06153-9
Acute kidney injury in critically ill patients with COVID-19
Abstract
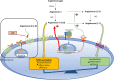
Acute kidney injury (AKI) has been reported in up to 25% of critically-ill patients with SARS-CoV-2 infection, especially in those with underlying comorbidities. AKI is associated with high mortality rates in this setting, especially when renal replacement therapy is required. Several studies have highlighted changes in urinary sediment, including proteinuria and hematuria, and evidence of urinary SARS-CoV-2 excretion, suggesting the presence of a renal reservoir for the virus. The pathophysiology of COVID-19 associated AKI could be related to unspecific mechanisms but also to COVID-specific mechanisms such as direct cellular injury resulting from viral entry through the receptor (ACE2) which is highly expressed in the kidney, an imbalanced renin-angotensin-aldosteron system, pro-inflammatory cytokines elicited by the viral infection and thrombotic events. Non-specific mechanisms include haemodynamic alterations, right heart failure, high levels of PEEP in patients requiring mechanical ventilation, hypovolemia, administration of nephrotoxic drugs and nosocomial sepsis. To date, there is no specific treatment for COVID-19 induced AKI. A number of investigational agents are being explored for antiviral/immunomodulatory treatment of COVID-19 and their impact on AKI is still unknown. Indications, timing and modalities of renal replacement therapy currently rely on non-specific data focusing on patients with sepsis. Further studies focusing on AKI in COVID-19 patients are urgently warranted in order to predict the risk of AKI, to identify the exact mechanisms of renal injury and to suggest targeted interventions.
Keywords: Acute kidney injury; COVID-19; Intensive care unit; Renin–angiotensin–aldosterone system.
Conflict of interest statement
Pr Darmon received funding from Sanofi (travel), Gilead-Kite (consulting and speaker), Astellas (speaker), Astute Medical (research support) and MSD (research support and speaker). Pr Azoulay has received fees for lectures from MSD, Pfizer and Alexion; his institution and research group have received support from Baxter, Jazz Pharmaceuticals, Fisher & Paykel, Gilead, Alexion and Ablynx; and he received support for article research from Assistance Publique-Hôpitaux de Paris. Pr. Zafrani’s institution received funding from the Assistance Publique Hôpitaux de Paris, Jazz Pharmaceuticals. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

