Evidence-based diagnostic use of VEMPs : From neurophysiological principles to clinical application
- PMID: 32533211
- PMCID: PMC7403168
- DOI: 10.1007/s00106-019-00767-2
Evidence-based diagnostic use of VEMPs : From neurophysiological principles to clinical application
Abstract
Background: Vestibular evoked myogenic potentials (VEMPs) are increasingly being used for testing otolith organ function.
Objective: This article provides an overview of the anatomical, biomechanical and neurophysiological principles underlying the evidence-based clinical application of ocular and cervical VEMPs (oVEMPs and cVEMPs).
Material and methods: Systematic literature search in PubMed until April 2019.
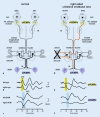
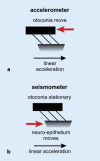
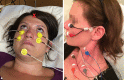
Results: Sound and vibration at a frequency of 500 Hz represent selective vestibular stimuli for the otolith organs. The predominant specificity of oVEMPs for contralateral utricular function and of cVEMPs for ipsilateral saccular function is defined by the different central projections of utricular and saccular afferents. VEMPs are particularly useful in the diagnosis of superior canal dehiscence and otolith organ specific vestibular dysfunction and as an alternative diagnostic approach in situations when video oculography is not possible or useful.
Conclusion: The use of VEMPs is a simple, safe, reliable and selective test of dynamic function of otolith organs.
Keywords: Bone conduction; Otolithic membrane; Vestibular evoked myogenic potentials; Vestibular neuritis; Vibration.
Conflict of interest statement
J. Dlugaiczyk received reimbursement of travel and congress fees by Hennig and Otometrics, speaker honoraria by Otometrics and research grants by Otonomy outside this study.
Figures

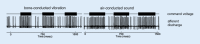

Similar articles
-
[Evidence-based diagnostic use of VEMPs : From neurophysiological principles to clinical application. German version].HNO. 2020 May;68(5):324-335. doi: 10.1007/s00106-019-00757-4. HNO. 2020. PMID: 31578599 Review. German.
-
Otolithic Receptor Mechanisms for Vestibular-Evoked Myogenic Potentials: A Review.Front Neurol. 2018 May 25;9:366. doi: 10.3389/fneur.2018.00366. eCollection 2018. Front Neurol. 2018. PMID: 29887827 Free PMC article. Review.
-
Ocular Vestibular Evoked Myogenic Potentials: Where Are We Now?Otol Neurotol. 2017 Dec;38(10):e513-e521. doi: 10.1097/MAO.0000000000001478. Otol Neurotol. 2017. PMID: 29135871 Review.
-
Clinical utility of ocular vestibular-evoked myogenic potentials (oVEMPs).Curr Neurol Neurosci Rep. 2015 May;15(5):22. doi: 10.1007/s11910-015-0548-y. Curr Neurol Neurosci Rep. 2015. PMID: 25773001 Review.
-
Otolith Function Testing.Adv Otorhinolaryngol. 2019;82:47-55. doi: 10.1159/000490271. Epub 2019 Jan 15. Adv Otorhinolaryngol. 2019. PMID: 30947185 Review.
Cited by
-
Bone conduction stimulated VEMPs by using the B250 transducer to assess the nerve of origin of sporadic vestibular schwannomas.Sci Rep. 2024 Nov 3;14(1):26483. doi: 10.1038/s41598-024-78060-8. Sci Rep. 2024. PMID: 39489829 Free PMC article.
-
The effect of Zexie decoction on vestibular and auditory function in DDAVP-induced endolymphatic hydrops of Guinea pigs.Front Neurol. 2025 Feb 24;16:1430522. doi: 10.3389/fneur.2025.1430522. eCollection 2025. Front Neurol. 2025. PMID: 40066303 Free PMC article.
-
Vestibular Hypersensitivity in Patients with Chronic Noise Exposure.Indian J Otolaryngol Head Neck Surg. 2022 Dec;74(Suppl 3):3957-3964. doi: 10.1007/s12070-021-02741-3. Epub 2021 Jul 21. Indian J Otolaryngol Head Neck Surg. 2022. PMID: 36742751 Free PMC article.
-
Skull vibration induced nystagmus, velocity storage and self-stability.Front Neurol. 2025 Feb 4;16:1533842. doi: 10.3389/fneur.2025.1533842. eCollection 2025. Front Neurol. 2025. PMID: 39968451 Free PMC article.
-
Vestibular syndromes after COVID-19 vaccination: A prospective cohort study.Eur J Neurol. 2022 Dec;29(12):3693-3700. doi: 10.1111/ene.15546. Epub 2022 Sep 21. Eur J Neurol. 2022. PMID: 36056895 Free PMC article.
References
-
- Akin FW, Murnane OD, Hall CD, Riska KM. Vestibular consequences of mild traumatic brain injury and blast exposure: a review. Brain Inj. 2017;31:1188–1194. - PubMed
-
- Burgess AM, Mezey LE, Manzari L, et al. Effect of stimulus rise-time on the ocular vestibular-evoked myogenic potential to bone-conducted vibration. Ear Hear. 2013;34:799–805. - PubMed
-
- Carey JP, Hirvonen TP, Hullar TE, et al. Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol Neurotol. 2004;25:345–352. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

