Resveratrol attenuates angiotensin II-induced cellular hypertrophy through the inhibition of CYP1B1 and the cardiotoxic mid-chain HETE metabolites
- PMID: 32533462
- PMCID: PMC7291180
- DOI: 10.1007/s11010-020-03777-9
Resveratrol attenuates angiotensin II-induced cellular hypertrophy through the inhibition of CYP1B1 and the cardiotoxic mid-chain HETE metabolites
Abstract
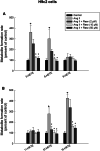
Several reports demonstrated the direct contribution of cytochrome P450 1B1 (CYP1B1) enzyme and its associated cardiotoxic mid-chain, hydroxyeicosatetraenoic acid (HETEs) metabolites in the development of cardiac hypertrophy. Resveratrol is commercially available polyphenol that exerts beneficial effects in wide array of cardiovascular diseases including cardiac hypertrophy, myocardial infarction and heart failure. Nevertheless, the underlying mechanisms responsible for these effects are not fully elucidated. Since resveratrol is a well-known CYP1B1 inhibitor, the purpose of this study is to test whether resveratrol attenuates angiotensin II (Ang II)-induced cellular hypertrophy through inhibition of CYP1B1/mid-chain HETEs mechanism. RL-14 and H9c2 cells were treated with vehicle or 10 μM Ang II in the absence and presence of 2, 10 or 50 μM resveratrol for 24 h. Thereafter, the level of mid-chain HETEs was determined using liquid chromatography-mass spectrometry (LC/MS). Hypertrophic markers and CYP1B1 gene expression and protein levels were measured using real-time PCR and Western blot analysis, respectively. Our results demonstrated that resveratrol, at concentrations of 10 and 50 μM, was able to attenuate Ang-II-induced cellular hypertrophy as evidenced by substantial inhibition of hypertrophic markers, β-myosin heavy chain (MHC)/α-MHC and atrial natriuretic peptide. Ang II significantly induced the protein expression of CYP1B1 and increased the metabolite formation rate of its associated mid-chain HETEs. Interestingly, the protective effect of resveratrol was associated with a significant decrease of CYP1B1 protein expression and mid-chain HETEs. Our results provided the first evidence that resveratrol protects against Ang II-induced cellular hypertrophy, at least in part, through CYP1B1/mid-chain HETEs-dependent mechanism.
Keywords: CYP1B1; Cardiac hypertrophy; Mid-chain HETEs; Resveratrol.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
The role of cytochrome P450 1B1 and its associated mid-chain hydroxyeicosatetraenoic acid metabolites in the development of cardiac hypertrophy induced by isoproterenol.Mol Cell Biochem. 2017 May;429(1-2):151-165. doi: 10.1007/s11010-017-2943-y. Epub 2017 Mar 1. Mol Cell Biochem. 2017. PMID: 28251434
-
S-Enantiomer of 19-Hydroxyeicosatetraenoic Acid Preferentially Protects Against Angiotensin II-Induced Cardiac Hypertrophy.Drug Metab Dispos. 2018 Aug;46(8):1157-1168. doi: 10.1124/dmd.118.082073. Epub 2018 Jun 7. Drug Metab Dispos. 2018. PMID: 29880629
-
CYP1B1 inhibition attenuates doxorubicin-induced cardiotoxicity through a mid-chain HETEs-dependent mechanism.Pharmacol Res. 2016 Mar;105:28-43. doi: 10.1016/j.phrs.2015.12.016. Epub 2016 Jan 6. Pharmacol Res. 2016. PMID: 26772815
-
The role of mid-chain hydroxyeicosatetraenoic acids in the pathogenesis of hypertension and cardiac hypertrophy.Arch Toxicol. 2016 Jan;90(1):119-36. doi: 10.1007/s00204-015-1620-8. Epub 2015 Nov 2. Arch Toxicol. 2016. PMID: 26525395 Review.
-
Effect of inflammation on cytochrome P450-mediated arachidonic acid metabolism and the consequences on cardiac hypertrophy.Drug Metab Rev. 2023 Feb-May;55(1-2):50-74. doi: 10.1080/03602532.2022.2162075. Epub 2022 Dec 27. Drug Metab Rev. 2023. PMID: 36573379 Review.
Cited by
-
The Expression of Genes CYP1A1, CYP1B1, and CYP2J3 in Distinct Regions of the Heart and Its Possible Contribution to the Development of Hypertension.Biomedicines. 2024 Oct 17;12(10):2374. doi: 10.3390/biomedicines12102374. Biomedicines. 2024. PMID: 39457686 Free PMC article.
-
Effect of Methyl Glycoside on Apoptosis and Oxidative Stress in Hypoxia Induced-Reoxygenated H9C2 Cell Lines.Cell Biochem Biophys. 2025 Mar;83(1):1045-1056. doi: 10.1007/s12013-024-01539-8. Epub 2024 Sep 18. Cell Biochem Biophys. 2025. PMID: 39292425
-
Targeting arachidonic acid-related metabolites in COVID-19 patients: potential use of drug-loaded nanoparticles.Emergent Mater. 2021;4(1):265-277. doi: 10.1007/s42247-020-00136-8. Epub 2020 Nov 17. Emergent Mater. 2021. PMID: 33225219 Free PMC article. Review.
-
Sex-dependent alterations in cardiac cytochrome P450-mediated arachidonic acid metabolism in pressure overload-induced cardiac hypertrophy in rats.Drug Metab Dispos. 2025 May;53(5):100077. doi: 10.1016/j.dmd.2025.100077. Epub 2025 Mar 31. Drug Metab Dispos. 2025. PMID: 40273825 Free PMC article.
-
CYP1B1 as a therapeutic target in cardio-oncology.Clin Sci (Lond). 2020 Nov 13;134(21):2897-2927. doi: 10.1042/CS20200310. Clin Sci (Lond). 2020. PMID: 33185690 Free PMC article.
References
-
- 2016 Report on the Health of Canadians
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

