Rationale and design of an investigator-initiated, multicenter, prospective open-label, randomized trial to evaluate the effect of ipragliflozin on endothelial dysfunction in type 2 diabetes and chronic kidney disease: the PROCEED trial
- PMID: 32534578
- PMCID: PMC7293776
- DOI: 10.1186/s12933-020-01065-w
Rationale and design of an investigator-initiated, multicenter, prospective open-label, randomized trial to evaluate the effect of ipragliflozin on endothelial dysfunction in type 2 diabetes and chronic kidney disease: the PROCEED trial
Abstract
Background: Type 2 diabetes (T2D) is associated with renal impairment and vascular endothelial dysfunction. Therefore, this pathological connection is an important therapeutic target. Recent cardiovascular and renal outcome trials demonstrated that sodium glucose cotransporter 2 inhibitors (SGLT2is) consistently reduced the risks of cardiovascular and renal events and mortality in patients with T2D and various other background risks including chronic kidney disease (CKD). However, the precise mechanisms by which SGLT2is accords these therapeutic benefits remain uncertain. It is also unknown whether these SGLT2is-associated benefits are associated with the amelioration of endothelial dysfunction in patients with T2D and CKD.
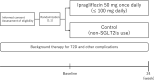
Methods: The PROCEED trial is an investigator-initiated, prospective, multicenter, open-label, randomized-controlled trial. The target sample size is 110 subjects. After they furnish informed consent and their endothelial dysfunction is confirmed from their decreased reactive hyperemia indices (RHI), eligible participants with T2D (HbA1c, 6.0-9.0%) and established CKD (30 mL/min/1.73 m2 ≤ estimated glomerular filtration ratio [eGFR] < 60 and/or ≥ urine albumin-to-creatinine ratio 30 mg/g Cr) will be randomized (1:1) to receive either 50 mg ipragliflozin daily or continuation of background treatment (non-SGLT2i). The primary endpoint is the change in RHI from baseline after 24 weeks. To compare the treatment effects between groups, the baseline-adjusted means and their 95% confidence intervals will be estimated by analysis of covariance adjusted for HbA1c (< 7.0% or ≥ 7.0%), age (< 70 y or ≥ 70 y), RHI (< 1.67 or ≥ 1.67), eGFR (< 45 mL/min/1.73 m2 or ≥ 45 mL/min/1.73 m2), and smoking status. Prespecified responder analyses will be also conducted to determine the proportions of patients with clinically meaningful changes in RHI at 24 weeks.
Discussion: PROCEED is the first trial to examine the effects of ipragliflozin on endothelial dysfunction in patients with T2D and CKD. This ongoing trial will establish whether endothelial dysfunction is a therapeutic target of SGLT2is in this population. It will also provide deep insights into the potential mechanisms by which SGLT2is reduced the risks of cardiovascular and renal events in recent outcome trials. Trial registration Unique Trial Number, jRCTs071190054 (https://jrct.niph.go.jp/en-latest-detail/jRCTs071190054).
Keywords: Chronic kidney disease (CKD); Endothelial dysfunction; Ipragliflozin; Reactive hyperemia peripheral arterial tonometry (RH-PAT); Sodium glucose cotransporter 2 inhibitor (SGLT2i); Type 2 diabetes (T2D).
Conflict of interest statement
AT received modest honoraria from Ono, Kowa, Daiichi Sankyo, Taisho Toyama, Takeda, Mitsubishi Tanabe, Teijin, Novo Nordisk, Bayer, Fukuda Denshi, and Boehringer Ingelheim, and a research grant from GlaxoSmithKline. MS received grants non-purpose research grants from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Eli Lilly, Kowa, Mitsubishi Tanabe, MSD, Novo Nordisk, Ono, Taisho Toyama, and Takeda; lecturer fees from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Eli Lilly, Kowa, Mitsubishi Tanabe, Mochida, MSD, Novo Nordisk, Ono, Taisho Toyama, and Takeda; and advisory board for Novo Nordisk. YO received lecture fees from Astellas, AstraZeneca, Ono, Mitsubishi Tanabe, Bayer, Novo Nordisk, Kowa, and Sanofi. KN received honoraria from Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Mitsubishi Tanabe, MSD, Ono, Otsuka, and Takeda, research grants from Asahi Kasei, Astellas, Bayer, Boehringer Ingelheim, Mitsubishi Tanabe, Teijin, and Terumo, and scholarships from Astellas, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Takeda, and Teijin. Other authors declare no potential competing interests.
Figures
References
-
- Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73–81. - PubMed
-
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet (London, England) 2013;382(9889):339–352. - PubMed
-
- Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. - PMC - PubMed
-
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

