Study protocol of the DUtch PARkinson Cohort (DUPARC): a prospective, observational study of de novo Parkinson's disease patients for the identification and validation of biomarkers for Parkinson's disease subtypes, progression and pathophysiology
- PMID: 32534583
- PMCID: PMC7293131
- DOI: 10.1186/s12883-020-01811-3
Study protocol of the DUtch PARkinson Cohort (DUPARC): a prospective, observational study of de novo Parkinson's disease patients for the identification and validation of biomarkers for Parkinson's disease subtypes, progression and pathophysiology
Abstract
Background: Parkinson's Disease (PD) is a heterogeneous, progressive neurodegenerative disorder which is characterized by a variety of motor and non-motor symptoms. To date, no disease modifying treatment for PD exists. Here, the study protocol of the Dutch Parkinson Cohort (DUPARC) is described. DUPARC is a longitudinal cohort study aimed at deeply phenotyping de novo PD patients who are treatment-naïve at baseline, to discover and validate biomarkers for PD progression, subtypes and pathophysiology.
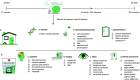
Methods/design: DUPARC is a prospective cohort study in which 150 de novo PD subjects will be recruited through a collaborative network of PD treating neurologists in the northern part of the Netherlands (Parkinson Platform Northern Netherlands, PPNN). Participants will receive follow-up assessments after 1 year and 3 years, with the intention of an extended follow-up with 3 year intervals. Subjects are extensively characterized to primarily assess objectives within three major domains of PD: cognition, gastrointestinal function and vision. This includes brain magnetic resonance imaging (MRI); brain cholinergic PET-imaging with fluoroethoxybenzovesamicol (FEOBV-PET); brain dopaminergic PET-imaging with fluorodopa (FDOPA-PET); detailed neuropsychological assessments, covering all cognitive domains; gut microbiome composition; intestinal wall permeability; optical coherence tomography (OCT); genotyping; motor and non-motor symptoms; overall clinical status and lifestyle factors, including a dietary assessment; storage of blood and feces for additional analyses of inflammation and metabolic parameters. Since the start of the inclusion, at the end of 2017, over 100 PD subjects with a confirmed dopaminergic deficit on FDOPA-PET have been included.
Discussion: DUPARC is the first study to combine data within, but not limited to, the non-motor domains of cognition, gastrointestinal function and vision in PD subjects over time. As a de novo PD cohort, with treatment naïve subjects at baseline, DUPARC provides a unique opportunity for biomarker discovery and validation without the possible confounding influences of dopaminergic medication.
Trial registration: NCT04180865; registered retrospectively, November 28th 2019.
Keywords: Biomarkers; Cognition; Gastrointestinal microbiome; Longitudinal studies; Microbiota; Neurodegenerative diseases; Neuropsychology; Observational study; Ophthalmology; Parkinson disease.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

