Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial
- PMID: 32534647
- PMCID: PMC7294238
- DOI: 10.1016/S0140-6736(20)30366-4
Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial
Abstract
Background: Lynch syndrome is associated with an increased risk of colorectal cancer and with a broader spectrum of cancers, especially endometrial cancer. In 2011, our group reported long-term cancer outcomes (mean follow-up 55·7 months [SD 31·4]) for participants with Lynch syndrome enrolled into a randomised trial of daily aspirin versus placebo. This report completes the planned 10-year follow-up to allow a longer-term assessment of the effect of taking regular aspirin in this high-risk population.
Methods: In the double-blind, randomised CAPP2 trial, 861 patients from 43 international centres worldwide (707 [82%] from Europe, 112 [13%] from Australasia, 38 [4%] from Africa, and four [<1%] from The Americas) with Lynch syndrome were randomly assigned to receive 600 mg aspirin daily or placebo. Cancer outcomes were monitored for at least 10 years from recruitment with English, Finnish, and Welsh participants being monitored for up to 20 years. The primary endpoint was development of colorectal cancer. Analysis was by intention to treat and per protocol. The trial is registered with the ISRCTN registry, number ISRCTN59521990.
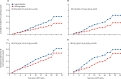
Findings: Between January, 1999, and March, 2005, 937 eligible patients with Lynch syndrome, mean age 45 years, commenced treatment, of whom 861 agreed to be randomly assigned to the aspirin group or placebo; 427 (50%) participants received aspirin and 434 (50%) placebo. Participants were followed for a mean of 10 years approximating 8500 person-years. 40 (9%) of 427 participants who received aspirin developed colorectal cancer compared with 58 (13%) of 434 who received placebo. Intention-to-treat Cox proportional hazards analysis revealed a significantly reduced hazard ratio (HR) of 0·65 (95% CI 0·43-0·97; p=0·035) for aspirin versus placebo. Negative binomial regression to account for multiple primary events gave an incidence rate ratio of 0·58 (0·39-0·87; p=0·0085). Per-protocol analyses restricted to 509 who achieved 2 years' intervention gave an HR of 0·56 (0·34-0·91; p=0·019) and an incidence rate ratio of 0·50 (0·31-0·82; p=0·0057). Non-colorectal Lynch syndrome cancers were reported in 36 participants who received aspirin and 36 participants who received placebo. Intention-to-treat and per-protocol analyses showed no effect. For all Lynch syndrome cancers combined, the intention-to-treat analysis did not reach significance but per-protocol analysis showed significantly reduced overall risk for the aspirin group (HR=0·63, 0·43-0·92; p=0·018). Adverse events during the intervention phase between aspirin and placebo groups were similar, and no significant difference in compliance between intervention groups was observed for participants with complete intervention phase data; details reported previously.
Interpretation: The case for prevention of colorectal cancer with aspirin in Lynch syndrome is supported by our results.
Funding: Cancer Research UK, European Union, MRC, NIHR, Bayer Pharma AG, Barbour Foundation.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Aspirin for Lynch syndrome: a legacy of prevention.Lancet. 2020 Jun 13;395(10240):1817-1818. doi: 10.1016/S0140-6736(20)30973-9. Lancet. 2020. PMID: 32534636 No abstract available.
References
-
- Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399–4404. - PubMed
-
- Cuzick J, Otto F, Baron JA. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. - PubMed
-
- Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

