Animal toxins - Nature's evolutionary-refined toolkit for basic research and drug discovery
- PMID: 32535105
- PMCID: PMC7290223
- DOI: 10.1016/j.bcp.2020.114096
Animal toxins - Nature's evolutionary-refined toolkit for basic research and drug discovery
Abstract
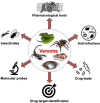
Venomous animals have evolved toxins that interfere with specific components of their victim's core physiological systems, thereby causing biological dysfunction that aids in prey capture, defense against predators, or other roles such as intraspecific competition. Many animal lineages evolved venom systems independently, highlighting the success of this strategy. Over the course of evolution, toxins with exceptional specificity and high potency for their intended molecular targets have prevailed, making venoms an invaluable and almost inexhaustible source of bioactive molecules, some of which have found use as pharmacological tools, human therapeutics, and bioinsecticides. Current biomedically-focused research on venoms is directed towards their use in delineating the physiological role of toxin molecular targets such as ion channels and receptors, studying or treating human diseases, targeting vectors of human diseases, and treating microbial and parasitic infections. We provide examples of each of these areas of venom research, highlighting the potential that venom molecules hold for basic research and drug development.
Keywords: Drug discovery; Insecticides; Pharmacological tools; Therapeutics; Venom; Venom peptides.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Fry B.G., Roelants K., Champagne D.E., Scheib H., Tyndall J.D., King G.F. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 2009;10:483–511. - PubMed
-
- Baron A., Diochot S., Salinas M., Deval E., Noel J., Lingueglia E. Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid-sensing ion channels. Toxicon. 2013;75:187–204. - PubMed
-
- Dutertre S., Nicke A., Tsetlin V.I. Nicotinic acetylcholine receptor inhibitors derived from snake and snail venoms. Neuropharmacology. 2017;127:196–223. - PubMed
-
- Olivera B.M., Miljanich G.P., Ramachandran J., Adams M.E. Calcium channel diversity and neurotransmitter release: the ω-conotoxins and ω-agatoxins. Annu. Rev. Biochem. 1994;63:823–867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

