DRH1 - a novel blood-based HPV tumour marker
- PMID: 32535546
- PMCID: PMC7300133
- DOI: 10.1016/j.ebiom.2020.102804
DRH1 - a novel blood-based HPV tumour marker
Abstract
Background: To date, no studies have successfully shown that a highly specific, blood-based tumour marker to detect clinically relevant HPV-induced disease could be used for screening, monitoring therapy response or early detection of recurrence. This study aims to assess the clinical performance of a newly developed HPV16-L1 DRH1 epitope-specific serological assay.
Methods: In a multi-centre study sera of 1486 patients (301 Head and Neck Squamous Cell Carcinoma (HNSCC) patients, 12 HIV+ anal cancer patients, 80 HIV-positive patients, 29 Gardasil-9-vaccinees, 1064 healthy controls) were tested for human HPV16-L1 DRH1 antibodies. Analytical specificity was determined using WHO reference-sera for HPV16/18 and 29 pre- and post-immune sera of Gardasil-9-vaccinees. Tumour-tissue was immunochemically stained for HPV-L1-capsidprotein-expression.
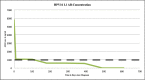
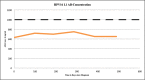
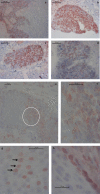
Findings: The DRH1-competitive-serological-assay showed a sensitivity of 95% (95% CI, 77.2-99.9%) for HPV16-driven HNSCC, and 90% (95% CI, 55.5-99.7%) for HPV16-induced anal cancer in HIV-positives. Overall diagnostic specificity was 99.46% for men and 99.29% for women ≥ 30 years. After vaccination, antibody level increased from average 364 ng/ml to 37,500 ng/ml. During post-therapy-monitoring, HNSCC patients showing an antibody decrease in the range of 30-100% lived disease free over a period of up to 26 months. The increase of antibodies from 2750 to 12,000 ng/ml mirrored recurrent disease. We can also show that the L1-capsidprotein is expressed in HPV16-DNA positive tumour-tissue.
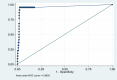
Interpretation: HPV16-L1 DRH1 epitope-specific antibodies are linked to HPV16-induced malignant disease. As post-treatment biomarker, the assay allows independent post-therapy monitoring as well as early diagnosis of tumour recurrence. An AUC of 0.96 indicates high sensitivity and specificity for early detection of HPV16-induced disease.
Funding: The manufacturer provided assays free of charge.
Keywords: Antibodies; Blood test; HNSCC; HPV16; Screening; Tumour marker.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All participating authors hereby disclose any financial and personal relationships with other people or organisations that could have inappropriately influenced the current study.
Figures


References
-
- de Villiers E.M., Fauquet C., Broker T.R., Bernard H.U., zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. - PubMed
-
- Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

