IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy?
- PMID: 32535732
- PMCID: PMC7292936
- DOI: 10.1007/s40290-020-00342-z
IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy?
Abstract
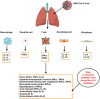
At present, there are no proven agents for treatment of coronavirus disease (COVID-19). The available evidence has not allowed guidelines to clearly recommend any drugs outside the context of clinical trials. The novel coronavirus SARS-CoV-2 that causes COVID-19 invokes a hyperinflammatory state driven by multiple cells and mediators like interleukin (IL)-1, IL-6, IL-12, and IL-18, tumor necrosis factor alpha (TNFα), etc. Considering the proven role of cytokine dysregulation in causing this hyperinflammation in the lungs with IL-6 being a key driver, particularly in seriously ill COVID-19 patients, it is crucial to further explore selective cytokine blockade with drugs like the IL-6 inhibitors tocilizumab, sarilumab, and siltuximab. These targeted monoclonal antibodies can dampen the downstream IL-6 signaling pathways, which can lead to decreased cell proliferation, differentiation, oxidative stress, exudation, and improve clinical outcomes in patients with evident features of cytokine-driven inflammation like persistent fever, dyspnea and elevated markers. Preliminary evidence has come for tocilizumab from some small studies, and interim analysis of a randomized controlled trial; the latter also being available for sarilumab. International guidelines do include IL-6 inhibitors as one of the options available for severe or critically ill patients. There has been increased interest in evaluating these drugs with a series of clinical trials being registered and conducted in different countries. The level of investigation though perhaps needs to be further intensified as there is a need to focus on therapeutic options that can prove to be 'life-saving' as the number of COVID-19 fatalities worldwide keeps increasing alarmingly. IL-6 inhibitors could be one such treatment option, with generation of more evidence and completion of a larger number of systematic studies.
Conflict of interest statement
None.
Figures
References
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html. Accessed 28 May 2020.
-
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [JAMA Network]. Available at: https://jamanetwork.com/journals/jama/fullarticle/2763667. Accessed 29 Apr 2020. - PubMed
-
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med [Epub ahead of print]. 2020;1–4. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7131986/. Accessed 29 Apr 2020. - PMC - PubMed
-
- Conti P, Gallenga CE, Tetè G, Caraffa A, Ronconi G, Younes A, et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J Biol Regul Homeost Agents [Epub ahead of print]. 2020;34(2). Available at: https://pubmed.ncbi.nlm.nih.gov/32228825/?from_term=Caraffa+A&from_cauth.... Accessed 29 May 2020. - PubMed
-
- Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents [Epub ahead of print]. 2020;34(2). Available at: https://www.biolifesas.org/biolife/2020/04/07/coronavirus-cov-19-sars-co.... Accessed 29 May 2020. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous