The lung ultrasound: facts or artifacts? In the era of COVID-19 outbreak
- PMID: 32535787
- PMCID: PMC7293437
- DOI: 10.1007/s11547-020-01236-5
The lung ultrasound: facts or artifacts? In the era of COVID-19 outbreak
Abstract
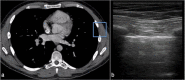
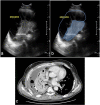
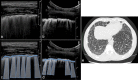
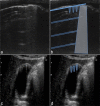
Ultrasound is the most disruptive innovation in intensive care life, above all in this time, with a high diagnostic value when applied appropriately. In recent years, point-of-care lung ultrasound has gained significant popularity as a diagnostic tool in the acutely dyspnoeic patients. In the era of Sars-CoV-2 outbreak, lung ultrasound seems to be strongly adapting to the follow-up for lung involvement of patients with ascertaining infections, till to be used, in our opinion emblematically, as a screening test in suspected patients at the emergency triage or at home medical visit. In this brief review, we discuss the lung ultrasound dichotomy, certainties and uncertainties, describing its potential role in validated clinical contexts, as a clinical-dependent exam, its limits and pitfalls in a generic and off-label clinical context, as a virtual anatomical-dependent exam, and its effects on the clinical management of patients with COVID-19.
Keywords: COVID-19; Dyspnoea; Lung ultrasound; Point of care ultrasound; Sars-CoV-2.
Conflict of interest statement
The Authors declare that they have no conflict of interest.
Figures
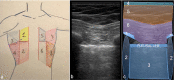

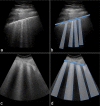
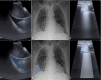
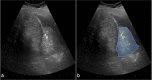






References
-
- Secco G, Delorenzo M, Zattera C, Giacomuzzi Moore B, Demitry L, Vezzoni G, Resta F, Barcella B, Cappa G, Perrone T, Salinaro F, Perlini S. Lung ultrasound in COVID-19: a useful diagnostic tool. Emer Care J. 2020 doi: 10.4081/ecj.2020.9017. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

