Image guidance in radiation therapy for better cure of cancer
- PMID: 32536001
- PMCID: PMC7332209
- DOI: 10.1002/1878-0261.12751
Image guidance in radiation therapy for better cure of cancer
Abstract
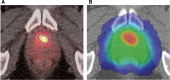
The key goal and main challenge of radiation therapy is the elimination of tumors without any concurring damages of the surrounding healthy tissues and organs. Radiation doses required to achieve sufficient cancer-cell kill exceed in most clinical situations the dose that can be tolerated by the healthy tissues, especially when large parts of the affected organ are irradiated. High-precision radiation oncology aims at optimizing tumor coverage, while sparing normal tissues. Medical imaging during the preparation phase, as well as in the treatment room for localization of the tumor and directing the beam, referred to as image-guided radiotherapy (IGRT), is the cornerstone of precision radiation oncology. Sophisticated high-resolution real-time IGRT using X-rays, computer tomography, magnetic resonance imaging, or ultrasound, enables delivery of high radiation doses to tumors without significant damage of healthy organs. IGRT is the most convincing success story of radiation oncology over the last decades, and it remains a major driving force of innovation, contributing to the development of personalized oncology, for example, through the use of real-time imaging biomarkers for individualized dose delivery.
Keywords: MR-linac; adaptive radiotherapy; brachytherapy; cone-beam CT; image guidance; molecular imaging; radiation; stereotactic radiotherapy.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
DZ and DT receive financial and technical support from Elekta AB (Stockholm, Sweden) under a research agreement. For the MRgRT program in Tübingen, DZ and DT receive funding by the German Research Council (PAK 997/1, ZI 736/2‐1), University Hospital Tübingen, and Medical Faculty Tübingen. DZ and DT receive sponsoring for travels and scientific symposia from Elekta, Siemens, Philips, and Dr. Sennewald. DZ and DT confirm that none of the above‐mentioned funding sources were involved in the study design, in the collection, analysis, and interpretation of data and in the writing of the paper. The department of Radiation Oncology at Amsterdam UMC received research support from Varian medical systems and Viewray Inc.
Figures




References
-
- Herman MG, Balter JM, Jaffray DA, McGee KP, Munro P, Shalev S, Van Herk M & Wong JW (2001) Clinical use of electronic portal imaging: report of AAPM radiation therapy committee task group 58. Med Phys 28, 712–737. - PubMed
-
- Shimizu S, Shirato H, Kitamura K, Shinohara N, Harabayashi T, Tsukamoto T, Koyanagi T & Miyasaka K (2000) Use of an implanted marker and real‐time tracking of the marker for the positioning of prostate and bladder cancers. Int J Radiat Oncol Biol Phys 48, 1591–1597. - PubMed
-
- Court L, Rosen I, Mohan R & Dong L (2003) Evaluation of mechanical precision and alignment uncertainties for an integrated CT/LINAC system. Med Phys 30, 1198–1210. - PubMed
-
- Barker JL Jr, Garden AS, Ang KK, O'Daniel JC, Wang H, Court LE, Morrison WH, Rosenthal DI, Chao KS, Tucker SL et al (2004) Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head‐and‐neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys 59, 960–970. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

