Hemodynamic and Histopathological Changes in the Early Phase of the Development of an Intracranial Aneurysm
- PMID: 32536660
- PMCID: PMC7358784
- DOI: 10.2176/nmc.st.2020-0072
Hemodynamic and Histopathological Changes in the Early Phase of the Development of an Intracranial Aneurysm
Abstract
Hemodynamic stress and chronic inflammation are closely associated with the pathogenesis of intracranial aneurysms (IAs). However, the hemodynamic and biological mechanisms triggering IA formation remain to be elucidated. To clarify them, computational fluid dynamics (CFD) and histopathological analyses in the early phase of IA development using an experimentally induced IA model in rats were conducted. Histological changes in the early phase of IA development were observed under a scanning electron microscope (SEM) and a transmission electron microscope (TEM). Using data from 7-T magnetic resonance angiography (7T-MRA), CFD analyses were performed to determine wall shear stress (WSS) and wall pressure (WP) at the prospective site of IA. A bump-like protrusion named an "intimal pad" was located in the anterior cerebral artery (ACA) immediately distal to the apex of the bifurcation. TEM showed the degeneration of the internal elastic lamina (IEL) and longitudinally elongated smooth muscle cells (SMCs) that switched from the contractile to the proliferative phenotype and penetrated between two divided layers of the degenerated IEL in the prospective site of the IA. However, no inflammatory cells were observed. CFD analyses showed no particular pattern of WSS and WP at the prospective IA site. IEL degeneration and the phenotypic change and longitudinal elongation of SMCs were identified as the early events in IA development. CFD analyses and TEM data suggest that these biological events may be derived from increased circumferential wall stress due to increased blood pressure and increased longitudinal wall strain due to the existence of the intimal pad.
Keywords: hemodynamics; internal elastic lamina; intracranial aneurysm; smooth muscle cell.
Conflict of interest statement
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices in the article. H.K., T.I., K.Y., and S.M are members of The Japan Neurosurgical Society (JNS) and have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
Figures


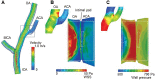

Similar articles
-
Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms.Neurosurg Focus. 2019 Jul 1;47(1):E21. doi: 10.3171/2019.5.FOCUS19234. Neurosurg Focus. 2019. PMID: 31261126 Free PMC article. Review.
-
Dedifferentiation of smooth muscle cells in intracranial aneurysms and its potential contribution to the pathogenesis.Sci Rep. 2020 May 20;10(1):8330. doi: 10.1038/s41598-020-65361-x. Sci Rep. 2020. PMID: 32433495 Free PMC article.
-
The biophysical role of hemodynamics in the pathogenesis of cerebral aneurysm formation and rupture.Neurosurg Focus. 2019 Jul 1;47(1):E11. doi: 10.3171/2019.4.FOCUS19232. Neurosurg Focus. 2019. PMID: 31261115 Review.
-
Two Diverse Hemodynamic Forces, a Mechanical Stretch and a High Wall Shear Stress, Determine Intracranial Aneurysm Formation.Transl Stroke Res. 2020 Feb;11(1):80-92. doi: 10.1007/s12975-019-0690-y. Epub 2019 Feb 8. Transl Stroke Res. 2020. PMID: 30737656
-
Local hemodynamics at the rupture point of cerebral aneurysms determined by computational fluid dynamics analysis.Cerebrovasc Dis. 2012;34(2):121-9. doi: 10.1159/000339678. Epub 2012 Aug 1. Cerebrovasc Dis. 2012. PMID: 22965244
Cited by
-
A novel clinical-radscore nomogram for predicting ruptured intracranial aneurysm.Heliyon. 2023 Oct 5;9(10):e20718. doi: 10.1016/j.heliyon.2023.e20718. eCollection 2023 Oct. Heliyon. 2023. PMID: 37842571 Free PMC article.
-
Early vascular embolization improves neurological function in patients with intracranial aneurysm.Am J Transl Res. 2024 Apr 15;16(4):1273-1280. doi: 10.62347/ISIS6489. eCollection 2024. Am J Transl Res. 2024. PMID: 38715829 Free PMC article.
-
Trauma may affect vasa vasorum to promote thrombosis and enlargement of intracranial aneurysms: A case report.Surg Neurol Int. 2021 Jan 13;12:16. doi: 10.25259/SNI_750_2020. eCollection 2021. Surg Neurol Int. 2021. PMID: 33500831 Free PMC article.
-
Association of bleb formation with peri-aneurysmal contact in unruptured intracranial aneurysms.Sci Rep. 2022 Apr 12;12(1):6075. doi: 10.1038/s41598-022-10064-8. Sci Rep. 2022. PMID: 35414058 Free PMC article.
-
The biology of bone morphogenetic protein signaling pathway in cerebrovascular system.Chin Neurosurg J. 2021 Sep 1;7(1):36. doi: 10.1186/s41016-021-00254-0. Chin Neurosurg J. 2021. PMID: 34465399 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

