Diverse Virus and Host-Dependent Mechanisms Influence the Systemic and Intrahepatic Immune Responses in the Woodchuck Model of Hepatitis B
- PMID: 32536912
- PMCID: PMC7267019
- DOI: 10.3389/fimmu.2020.00853
Diverse Virus and Host-Dependent Mechanisms Influence the Systemic and Intrahepatic Immune Responses in the Woodchuck Model of Hepatitis B
Abstract
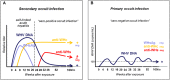
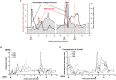
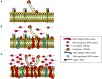
Woodchuck infected with woodchuck hepatitis virus (WHV) represents the pathogenically nearest model of hepatitis B and associated hepatocellular carcinoma (HCC). This naturally occurring animal model also is highly valuable for development and preclinical evaluation of new anti-HBV agents and immunotherapies against chronic hepatitis (CH) B and HCC. Studies in this system uncovered a number of molecular and immunological processes which contribute or likely contribute to the immunopathogenesis of liver disease and modulation of the systemic and intrahepatic innate and adaptive immune responses during hepadnaviral infection. Among them, inhibition of presentation of the class I major histocompatibility complex on chronically infected hepatocytes and a role of WHV envelope proteins in this process, as well as augmented hepatocyte cytotoxicity mediated by constitutively expressed components of CD95 (Fas) ligand- and perforin-dependent pathways, capable of eliminating cells brought to contact with hepatocyte surface, including activated T lymphocytes, were uncovered. Other findings pointed to a role of autoimmune response against hepatocyte asialoglycoprotein receptor in augmenting severity of liver damage in hepadnaviral CH. It was also documented that WHV in the first few hours activates intrahepatic innate immunity that transiently decreases hepatic virus load. However, this activation is not translated in a timely manner to induction of virus-specific T cell response which appears to be hindered by defective activation of antigen presenting cells and presentation of viral epitopes to T cells. The early WHV infection also induces generalized polyclonal activation of T cells that precedes emergence of virus-specific T lymphocyte reactivity. The combination of these mechanisms hinder recognition of virus allowing its dissemination in the initial, asymptomatic stages of infection before adaptive cellular response became apparent. This review will highlight a range of diverse mechanisms uncovered in the woodchuck model which affect effectiveness of the anti-viral systemic and intrahepatic immune responses, and modify liver disease outcomes. Further exploration of these and other mechanisms, either already discovered or yet unknown, and their interactions should bring more comprehensive understanding of HBV pathogenesis and help to identify novel targets for therapeutic and preventive interventions. The woodchuck model is uniquely positioned to further contribute to these advances.
Keywords: asialoglycoprotein receptor; hepatocyte as cytotoxic immune effector; intrahepatic innate and adaptive immune responses; major histocompatibility complex presentation; pre-acute infection; toll-like receptors; virus-hepatocyte interaction; woodchuck model of hepatitis B.
Copyright © 2020 Michalak.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

