Analysis of the Targets and Glycosylation of Monoclonal IgAs From MGUS and Myeloma Patients
- PMID: 32536913
- PMCID: PMC7266999
- DOI: 10.3389/fimmu.2020.00854
Analysis of the Targets and Glycosylation of Monoclonal IgAs From MGUS and Myeloma Patients
Abstract
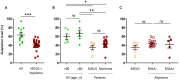
Previous studies showed that monoclonal immunoglobulins G (IgGs) of "monoclonal gammopathy of undetermined significance" (MGUS) and myeloma were hyposialylated, thus presumably pro-inflammatory, and for about half of patients, the target of the monoclonal IgG was either a virus-Epstein-Barr virus (EBV), other herpes viruses, hepatitis C virus (HCV)-or a glucolipid, lysoglucosylceramide (LGL1), suggesting antigen-driven disease in these patients. In the present study, we show that monoclonal IgAs share these characteristics. We collected 35 sera of patients with a monoclonal IgA (6 MGUS, 29 myeloma), and we were able to purify 25 of the 35 monoclonal IgAs (6 MGUS, 19 myeloma). Monoclonal IgAs from MGUS and myeloma patients were significantly less sialylated than IgAs from healthy volunteers. When purified monoclonal IgAs were tested against infectious pathogens and LGL1, five myeloma patients had a monoclonal IgA that specifically recognized viral proteins: the core protein of HCV in one case, EBV nuclear antigen 1 (EBNA-1) in four cases (21.1% of IgA myeloma). Monoclonal IgAs from three myeloma patients reacted against LGL1. In summary, monoclonal IgAs are hyposialylated and as described for IgG myeloma, significant subsets (8/19, or 42%) of patients with IgA myeloma may have viral or self (LGL1) antigen-driven disease.
Keywords: Epstein–Barr virus; hepatitis C virus; infectious antigens; lysoglucosylceramide (LGL-1); monoclonal gammopathy of undetermined significance (MGUS); monoclonal immunoglobulin A (IgA); multiple myeloma; sialylation.
Copyright © 2020 Bosseboeuf, Seillier, Mennesson, Allain-Maillet, Fourny, Tallet, Piver, Lehours, Mégraud, Berthelot, Harb, Bigot-Corbel and Hermouet.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

