Association between Nod-like receptor protein 3 inflammasome and gouty nephropathy
- PMID: 32536991
- PMCID: PMC7281944
- DOI: 10.3892/etm.2020.8694
Association between Nod-like receptor protein 3 inflammasome and gouty nephropathy
Abstract
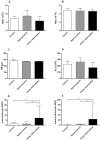
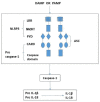
Crystalized deposits of monosodium urate activate the Nod-like receptor protein 3 (NLRP3) inflammasome, resulting in kidney damage. The present study investigated whether the NLRP3 inflammasome is associated with the progression of hyperuricaemia and gouty nephropathy. Adult male patients were recruited at the Affiliated Baoan Hospital of Shenzhen and divided into three groups of 15 patients each: The control group, the hyperuricaemia group and the gouty nephropathy group. General characteristics and organ function indicators were also measured for each patient. NLRP3, apoptosis-associated speck like protein (ASC) and caspase-1 mRNA and protein expressions in peripheral blood mononuclear cells were detected. The expression of certain downstream inflammatory factors, including interleukin (IL)-1β and IL-18 were also assessed in plasma. The results demonstrated that the concentration of uric acid and creatinine were increased in the hyperuricaemia and gouty nephropathy groups compared with the control group. NLRP3, ASC and caspase-1 mRNA and protein expression, and IL-1β and IL-18 expression were increased in the hyperuricaemia and gouty nephropathy groups compared with the control group. In addition, ASC and caspase-1 mRNA and protein expression, and IL-1β expression were higher in the gouty nephropathy group compared with the hyperuricaemia group. In conclusion, the present results supported the hypothesis that the NLRP3 inflammasome signalling pathway is associated with gouty nephropathy leading to initiation of the inflammatory response and causing renal damage.
Keywords: Nod-like receptor protein 3 inflammasome; gouty nephropathy; hyperuricaemia.
Copyright: © Zhang et al.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
