Osteogenic potential of poly(ethylene glycol)-amorphous calcium phosphate composites on human mesenchymal stem cells
- PMID: 32537121
- PMCID: PMC7268109
- DOI: 10.1177/2041731420926840
Osteogenic potential of poly(ethylene glycol)-amorphous calcium phosphate composites on human mesenchymal stem cells
Abstract
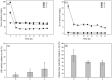
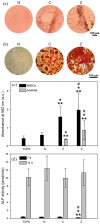
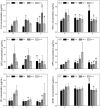
Synthetic hydrogel-amorphous calcium phosphate composites are promising candidates to substitute biologically sourced scaffolds for bone repair. While the hydrogel matrix serves as a template for stem cell colonisation, amorphous calcium phosphate s provide mechanical integrity with the potential to stimulate osteogenic differentiation. Here, we utilise composites of poly(ethylene glycol)-based hydrogels and differently stabilised amorphous calcium phosphate to investigate potential effects on attachment and osteogenic differentiation of human mesenchymal stem cells. We found that functionalisation with integrin binding motifs in the form of RGD tripeptide was necessary to allow adhesion of large numbers of cells in spread morphology. Slow dissolution of amorphous calcium phosphate mineral in the scaffolds over at least 21 days was observed, resulting in the release of calcium and zinc ions into the cell culture medium. While we qualitatively observed an increasingly mineralised extracellular matrix along with calcium deposition in the presence of amorphous calcium phosphate-loaded scaffolds, we did not observe significant changes in the expression of selected osteogenic markers.
Keywords: ACP; Hydrogel; PEG; calcium phosphate; cell differentiation; citrate; human mesenchymal stem cells; tissue engineering; zinc.
© The Author(s) 2020.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury 2005; 36(Suppl. 3): 20–27. - PubMed
-
- Laurencin CT, El-Amin SF. Xenotransplantation in orthopaedic surgery. J Am Acad Orthop Surg 2008; 16(1): 4–8. - PubMed
-
- Arrington ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res 1996; 329: 300–309. - PubMed
-
- Palmer SH, Gibbons CL, Athanasou NA. The pathology of bone allograft. J Bone Jt Surg Brit 1999; 81: 333–335. - PubMed
-
- Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am 1995; 77(11): 1742–1754. - PubMed
LinkOut - more resources
Full Text Sources

