Phase-dependent redox insulation in mussel adhesion
- PMID: 32537498
- PMCID: PMC7269650
- DOI: 10.1126/sciadv.aaz6486
Phase-dependent redox insulation in mussel adhesion
Abstract
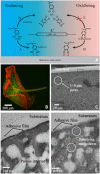
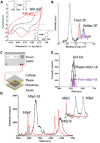
Catecholic 3,4-dihydroxyphenyl-l-alanine (Dopa) residues in mussel foot proteins (mfps) contribute critically to mussel (Mytilus californianus) plaque adhesion, but only if protected from oxidation at the adhesive-substratum interface. Dopa oxidation is thermodynamically favorable in seawater yet barely detectable in plaques; therefore, we investigated how plaques insulate Dopa-containing mfps against oxidation. Seawater sulfate triggers an mfp3 and mfp6 liquid-liquid phase separation (LLPS). By combining plaque cyclic voltammetry with electrophoresis, mass spectrometry, and redox-exchange chemistry, we show that Dopa-containing mfp3 and mfp6 in phase-separated droplets remain stable despite rapid oxidation in the surrounding equilibrium solution. The results suggest that a cohort of oxidation-prone proteins is endowed with phase-dependent redox stability. Moreover, in forming LLPS compartments, Dopa proteins become reservoirs of chemical energy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Hyman A. A., Weber C. A., Jülicher F., Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014). - PubMed
-
- Vrhovski B., Jensen S., Weiss A. S., Coacervation characteristics of recombinant human tropoelastin. FEBS Journal 250, 92–98 (1997). - PubMed
-
- Jin H.-J., Kaplan D. L., Mechanism of silk processing in insects and spiders. Nature 424, 1057–1061 (2003). - PubMed
-
- Tan Y. P., Hoon S., Guerette P. A., Wei W., Ghadban A., Hao C., Miserez A., Waite J. H., Infiltration of a chitin by protein coacervates defines the squid beak mechanical gradient. Nat. Chem. Biol. 11, 489–495 (2015). - PubMed
-
- Yamanaka M., Ishizaki Y., Nagakawa T., Taoka A., Fukumori Y., Purification and characterization of coacervate-forming cuticular proteins from Papilio xuthus pupae. Zoolog. Sci. 30, 534–542 (2013). - PubMed

