Noninvasive in vivo 3D bioprinting
- PMID: 32537512
- PMCID: PMC7269646
- DOI: 10.1126/sciadv.aba7406
Noninvasive in vivo 3D bioprinting
Abstract
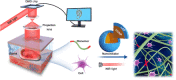
Three-dimensional (3D) printing technology has great potential in advancing clinical medicine. Currently, the in vivo application strategies for 3D-printed macroscale products are limited to surgical implantation or in situ 3D printing at the exposed trauma, both requiring exposure of the application site. Here, we show a digital near-infrared (NIR) photopolymerization (DNP)-based 3D printing technology that enables the noninvasive in vivo 3D bioprinting of tissue constructs. In this technology, the NIR is modulated into customized pattern by a digital micromirror device, and dynamically projected for spatially inducing the polymerization of monomer solutions. By ex vivo irradiation with the patterned NIR, the subcutaneously injected bioink can be noninvasively printed into customized tissue constructs in situ. Without surgery implantation, a personalized ear-like tissue constructs with chondrification and a muscle tissue repairable cell-laden conformal scaffold were obtained in vivo. This work provides a proof of concept of noninvasive in vivo 3D bioprinting.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures





References
-
- Derby B., Printing and prototyping of tissues and scaffolds. Science 338, 921–926 (2012). - PubMed
-
- Lee A., Hudson A. R., Shiwarski D. J., Tashman J. W., Hinton T. J., Yerneni S., Bliley J. M., Campbell P. G., Feinberg A. W., 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487 (2019). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

