Flux, toxicity, and expression costs generate complex genetic interactions in a metabolic pathway
- PMID: 32537514
- PMCID: PMC7269641
- DOI: 10.1126/sciadv.abb2236
Flux, toxicity, and expression costs generate complex genetic interactions in a metabolic pathway
Abstract
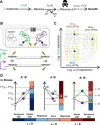
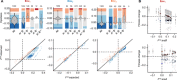
Our ability to predict the impact of mutations on traits relevant for disease and evolution remains severely limited by the dependence of their effects on the genetic background and environment. Even when molecular interactions between genes are known, it is unclear how these translate to organism-level interactions between alleles. We therefore characterized the interplay of genetic and environmental dependencies in determining fitness by quantifying ~4000 fitness interactions between expression variants of two metabolic genes, starting from various environmentally modulated expression levels. We detect a remarkable variety of interactions dependent on initial expression levels and demonstrate that they can be quantitatively explained by a mechanistic model accounting for catabolic flux, metabolite toxicity, and expression costs. Complex fitness interactions between mutations can therefore be predicted simply from their simultaneous impact on a few connected molecular phenotypes.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Scriver C. R., Waters P. J., Monogenic traits are not simple: Lessons from phenylketonuria. Trends Genet. 15, 267–272 (1999). - PubMed
-
- Badano J. L., Katsanis N., Beyond Mendel: An evolving view of human genetic disease transmission. Nat. Rev. Genet. 3, 779–789 (2002). - PubMed
-
- Manolio T. A., Collins F. S., Cox N. J., Goldstein D. B., Hindorff L. A., Hunter D. J., McCarthy M. I., Ramos E. M., Cardon L. R., Chakravarti A., Cho J. H., Guttmacher A. E., Kong A., Kruglyak L., Mardis E., Rotimi C. N., Slatkin M., Valle D., Whittemore A. S., Boehnke M., Clark A. G., Eichler E. E., Gibson G., Haines J. L., Mackay T. F. C., McCarroll S. A., Visscher P. M., Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009). - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

