Nanotechnology for angiogenesis: opportunities and challenges
- PMID: 32538379
- PMCID: PMC7418030
- DOI: 10.1039/c8cs01021h
Nanotechnology for angiogenesis: opportunities and challenges
Abstract
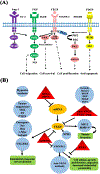
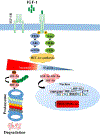
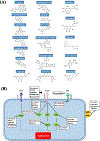
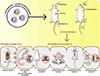
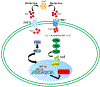
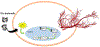
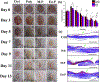
Angiogenesis plays a critical role within the human body, from the early stages of life (i.e., embryonic development) to life-threatening diseases (e.g., cancer, heart attack, stroke, wound healing). Many pharmaceutical companies have expended huge efforts on both stimulation and inhibition of angiogenesis. During the last decade, the nanotechnology revolution has made a great impact in medicine, and regulatory approvals are starting to be achieved for nanomedicines to treat a wide range of diseases. Angiogenesis therapies involve the inhibition of angiogenesis in oncology and ophthalmology, and stimulation of angiogenesis in wound healing and tissue engineering. This review aims to summarize nanotechnology-based strategies that have been explored in the broad area of angiogenesis. Lipid-based, carbon-based and polymeric nanoparticles, and a wide range of inorganic and metallic nanoparticles are covered in detail. Theranostic and imaging approaches can be facilitated by nanoparticles. Many preparations have been reported to have a bimodal effect where they stimulate angiogenesis at low dose and inhibit it at higher doses.
Figures






References
-
- Jain RK, Nature medicine, 2003, 9, 685. - PubMed
-
- Conway EM, Collen D and Carmeliet P, Cardiovascular research, 2001, 49, 507–521. - PubMed
-
- Carmeliet P and Jain RK, Nature, 2000, 407, 249–257. - PubMed
-
- Adams RH and Alitalo K, Nature reviews Molecular cell biology, 2007, 8, 464. - PubMed
-
- Schito L and Semenza GL, Trends in cancer, 2016, 2, 758–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

