Reporting of financial conflicts of interest by Canadian clinical practice guideline producers: a descriptive study
- PMID: 32538799
- PMCID: PMC7867217
- DOI: 10.1503/cmaj.191737
Reporting of financial conflicts of interest by Canadian clinical practice guideline producers: a descriptive study
Abstract
Background: The producers of clinical practice guidelines (CPGs) may not disclose industry funding in their CPGs. We reviewed Canadian national CPGs to examine the existence and disclosure of industry-related organizational funding in the CPGs, financial conflicts of interest of committee members and organizational procedures for managing financial conflicts of interest.
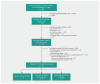
Methods: For this descriptive study, we searched the asset map of the Strategy for Patient-Oriented Research Evidence Alliance and the CPG Infobase for CPGs published between Jan. 1, 2016, and Nov. 30, 2018. Eligible guidelines had to have a national focus and either a first-line drug recommendation or a screening recommendation leading to drug treatment. One investigator reviewed all CPG titles to exclude those that were clearly ineligible. Two reviewers independently reviewed all remaining guidelines and extracted data. We analyzed the data descriptively.
Results: We included 21 CPGs: 3 from government-sponsored organizations, 9 from disease or condition interest groups and 9 from medical professional societies. None of the 3 government-sponsored organizations reported industry funding, and none of their committee members disclosed financial conflicts of interest. Among the 18 disease or condition interest groups and medical professional societies, 14 (93%) of the 15 that disclosed funding sources on websites (3 did not disclose) reported organizational funding from industry, but none disclosed this information in the CPGs; 12 (86%) of the 14 with conflict-of-interest disclosure statements in the CPG (4 did not include disclosures) had at least 1 committee member with a financial conflict (mean proportion of committee members with a conflict 56%); and for all 8 CPGs with identifiable chairs or cochairs (chairs or cochairs not reported for 10) at least 1 of these people had a financial conflict of interest. None of the guidelines described a plan to manage organizational financial conflicts of interest.
Interpretation: Canadian CPGs are vulnerable to industry influence through funding of producers of guidelines and through the financial conflicts of interest of committee members. The CPG producers that receive industry funding should disclose organizational financial conflicts in the CPGs, should engage independent oversight committees and should restrict voting on recommendations to guideline panelists who have no financial conflicts.
© 2020 Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Katharine Elder formerly worked as the administrator of the Canadian Task Force on Preventive Health Care (CTFPHC), which was the producer of one guideline included in the present study (cited as reference 41). Brett Thombs, Ainsley Moore and Sharon Straus are the chair, vice-chair and director of knowledge translation, respectively, for the CTFPHC. The present work was not commissioned or funded by the CTFPHC, and the authors participated in the study outside of their responsibilities with the CTFPHC. No other competing interests were declared.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
