Rampant Nuclear Transfer and Substitutions of Plastid Genes in Passiflora
- PMID: 32539116
- PMCID: PMC7488351
- DOI: 10.1093/gbe/evaa123
Rampant Nuclear Transfer and Substitutions of Plastid Genes in Passiflora
Abstract
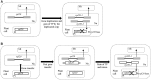
Gene losses in plastid genomes (plastomes) are often accompanied by functional transfer to the nucleus or substitution of an alternative nuclear-encoded gene. Despite the highly conserved gene content in plastomes of photosynthetic land plants, recent gene loss events have been documented in several disparate angiosperm clades. Among these lineages, Passiflora lacks several essential ribosomal genes, rps7, rps16, rpl20, rpl22, and rpl32, the two largest plastid genes, ycf1 and ycf2, and has a highly divergent rpoA. Comparative transcriptome analyses were performed to determine the fate of the missing genes in Passiflora. Putative functional transfers of rps7, rpl22, and rpl32 to nucleus were detected, with the nuclear transfer of rps7, representing a novel event in angiosperms. Plastid-encoded rps7 was transferred into the intron of a nuclear-encoded plastid-targeted thioredoxin m-type gene, acquiring its plastid transit peptide (TP). Plastid rpl20 likely experienced a novel substitution by a duplicated, nuclear-encoded mitochondrial-targeted rpl20 that has a similar gene structure. Additionally, among rosids, evidence for a third independent transfer of rpl22 in Passiflora was detected that gained a TP from a nuclear gene containing an organelle RNA recognition motif. Nuclear transcripts representing rpoA, ycf1, and ycf2 were not detected. Further analyses suggest that the divergent rpoA remains functional and that the gene is under positive or purifying selection in different clades. Comparative analyses indicate that alternative translocon and motor protein complexes may have substituted for the loss of ycf1 and ycf2 in Passiflora.
Keywords: ycf1/ycf2; gene loss; plastid-encoded RNA polymerase); plastid-encoded ribosomal genes; transit peptide.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures





References
-
- Adams KL, Palmer JD.. 2003. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol. 29(3):380–395. - PubMed
-
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc. Accessed June 5, 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

