Treatment with anagliptin, a DPP-4 inhibitor, decreases FABP4 concentration in patients with type 2 diabetes mellitus at a high risk for cardiovascular disease who are receiving statin therapy
- PMID: 32539832
- PMCID: PMC7296623
- DOI: 10.1186/s12933-020-01061-0
Treatment with anagliptin, a DPP-4 inhibitor, decreases FABP4 concentration in patients with type 2 diabetes mellitus at a high risk for cardiovascular disease who are receiving statin therapy
Abstract
Background: Fatty acid-binding protein 4 (FABP4) acts as a novel adipokine, and elevated FABP4 concentration is associated with obesity, insulin resistance and atherosclerosis. Dipeptidyl peptidase-4 (DPP-4) inhibitors, a class of antidiabetic drugs, have distinct structures among the drugs, possibly leading to a drug class effect and each drug effect. Sitagliptin, a DPP-4 inhibitor, has been reported to decrease FABP4 concentration in drug-naïve and sulfonylurea-treated patients with type 2 diabetes mellitus. Anagliptin, another DPP-4 inhibitor, was shown to decrease low-density lipoprotein cholesterol (LDL-C) level to a greater extent than that by sitagliptin in the Randomized Evaluation of Anagliptin vs. Sitagliptin On low-density lipoproteiN cholesterol in diabetes (REASON) trial.
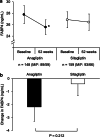
Aim and methods: As a sub-analysis study using data obtained from the REASON trial, we investigated the effects of treatment with anagliptin (n = 148, male/female: 89/59) and treatment with sitagliptin (n = 159, male/female: 93/66) for 52 weeks on FABP4 concentration in patients with type 2 diabetes mellitus at a high risk for cardiovascular events who were receiving statin therapy.
Results: The DPP-4 inhibitor had been administered in 82% of the patients in the anagliptin group and 81% of the patients in sitagliptin group prior to randomization. Serum FABP4 level was significantly decreased by 7.9% by treatment with anagliptin (P = 0.049) and was not significantly decreased by treatment with sitagliptin (P = 0.660). Change in FABP4 level was independently associated with basal FABP4 level and changes in waist circumference and creatinine after adjustment of age, sex and the treatment group.
Conclusion: Anagliptin decreases serum FABP4 concentration independent of change in hemoglobin A1c or LDL-C in patients with type 2 diabetes mellitus and dyslipidemia who are on statin therapy. Trial registration ClinicalTrials.gov number NCT02330406. Registered January 5, 2015, https://clinicaltrials.gov/ct2/show/NCT02330406.
Keywords: Anagliptin; Dipeptidyl peptidase-4 inhibitor; Fatty acid-binding protein; Sitagliptin.
Conflict of interest statement
MF. reports non-purpose research grants from Astellas, Mitsubishi Tanabe, Sanwa Kagaku Kenkyusho and MediciNova; lecturer’s fees from Mitsubishi Tanabe, Kowa, Mochida, Daiichi Sankyo, Novartis, Boehringer Ingelheim, MSD, Sanwa Kagaku Kenkyusho, Takeda, Astellas, Sanofi and AstraZeneca. I.S. reports research grants from Public Health Research Foundation, Kowa, National Cerebral and Cardiovascular Center and Medical Informatics Study Group; non-purpose research grants from Public Health Research Foundation, Eastep, Nexis, Takeda, Daiichi Sankyo, Beohringer Ingelheim, AstraZeneca, MSD, Amgen, Astellas, Sanofi, Fuji and Novartis; lecturer’s fees from AstraZeneca, Takeda, Bayer, Pfizer, Bristol-Myers Squibb, Boehringer lngelheim, MSD, Kyowa Hakko Kirin, Daiichi Sankyo, Novartis, Sanofi, Kowa, Shionogi, Kissei, Astellas, Amgen, Ono, Otsuka, Novonordisk, Mochida, Teijin, Sysmex, Nipro, Kyorin, Fuji and Sumitomo Dainippon; advisory board for Public Health Research Foundation, Kowa, Tanabe, Kyowa Hakko Kirin and Bristol-Myers Squibb, Sysmex. T.M. reports lecturer’s fees from Bayer, Daiichi Sankyo, Japan Lifeline, Kyocera, Mitsubishi Tanabe, Novartis, and Toray; manuscript fees from Bristol-Myers Squibb and Kowa; advisory boards for Asahi Kasei, Boston Scientific, and Bristol-Myers Squibb. Y.H., A.S. M.M. and M.Sa. declares no conflicts of interest. M.Sh. reports research grants from AstraZeneca, Ono, and Sanwa Kagaku Kenkyusho; non-purpose research grants from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Eli Lilly, Kowa, Mitsubishi Tanabe, MSD, Novo Nordisk, Ono, Taisho Toyama, and Takeda; lecturer’s fees from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Eli Lilly, Kowa, Mitsubishi Tanabe, Mochida, MSD, Novo Nordisk, Ono, Taisho Toyama, and Takeda; advisory board for Novo Nordisk; sponsored office from Boehringer Ingelheim. T.N. reports research grants from Eli Lilly, Mitsubishi Tanabe, MSD, and Novartis; lecturer’s fees from Arkray, Astellas, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Johnson & Johnson, Mitsubishi Tanabe, MSD, Novartis, Novo Nordisk, Ono, Sanofi, Sanwa Kagaku Kenkyusho, Sumitomo Dainippon, Taisho Toyama, Takeda, and Terumo. O.A. reports lecturer’s fees from Abbott, Astellas, Boehringer Ingelheim, Medtronic, and St. Jude Medical. K.N. reports research grants from Actelion, Asahi Kasei, Astellas, Astellas Amgen Bio Pharma, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Mitsubishi Tanabe, Novo Nordisk, Teijin, and Terumo; non-purpose research grants from Astellas, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, Japan Lifeline, Mitsubishi Tanabe, MSD, Novartis, Novo Nordisk, Ono, Otsuka, Pfizer, Sanofi, Sumitomo Dainippon, Takeda, and Teijin; lecturer’s fees from Actelion, Astellas, Astellas Amgen Bio Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Edwards Lifesciences, Eli Lilly, FUJIFILM, Fukuda Denshi, Kowa, Kyowa Hakko Kirin, Mebix, Medtronic, Mitsubishi Tanabe, Mochida, MSD, Novartis, Novo Nordisk, Ono, Otsuka, Pfizer, Roche Diagnostics, Sanofi, Sanwa Kagaku Kenkyusho, Sumitomo Dainippon, Taisho Toyama, Takeda, and Teijin; manuscript fee from Astellas, and Takeda; advisory board for Astellas, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Mitsubishi Tanabe, MSD, Novo Nordisk, Pfizer, and Takeda. S.U. reports research grants from Bristol-Myers Squibb, and Kowa; non-purpose research grants from Bristol-Myers Squibb, Chugai, MSD, Pfizer, and Takeda; lecturer’s fees from Boehringer Ingelheim, MSD, and Taiho; manuscript fees from Kowa; advisory board for Otsuka.
Figures

References
-
- Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–1379. - PubMed
-
- Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1(2):107–119. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

