Clinical outcomes and hemodynamic performance of Dafodil™ aortic and mitral pericardial bioprosthesis: 1-year results from Dafodil-1 first-in-human trial
- PMID: 32539847
- PMCID: PMC7294644
- DOI: 10.1186/s13019-020-01154-7
Clinical outcomes and hemodynamic performance of Dafodil™ aortic and mitral pericardial bioprosthesis: 1-year results from Dafodil-1 first-in-human trial
Abstract
Background: Bioprosthesis has been increasingly implanted for the treatment of transvalvular disease across the world. A new Dafodil™ pericardial bioprosthesis (Meril Life Sciences Pvt. Ltd., India) recently approved by Conformité Européenne (CE) is a tri-leaflet, stented, bovine valve. The purpose of Dafodil-1 first-in-human trial was to evaluate clinical safety and performance (including hemodynamic parameters) of the Dafodil pericardial bioprosthesis in patients who underwent aortic or mitral valve replacement.
Methods: This prospective, multicenter clinical trial enrolled 60 patients (Aortic: 30 patients; Mitral: 30 patients) from seven sites across India. Safety endpoints were early (≤30 days) and late (> 30 days) mortality and valve-related morbidity. The performance endpoints were hemodynamic performance, improvement in NYHA functional class, and change in the quality of life using SF-12v1 health survey.
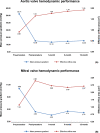
Results: From July 2017 to July 2018, 60 patients underwent implantation of the Dafodil pericardial bioprosthesis. Post-operatively, NYHA functional class significantly improved in all the patients (Aortic: 90% NYHA class-I and 10% NYHA class-II; Mitral: 96.55% NYHA class-I and 3.45% NYHA class-II; P < 0.001). There was no death in aortic valve replacement patients till 12-month. In mitral valve replacement patients, early mortalities occurred in three patients, and late mortality occurred in one patient; none of these were valve-related. Freedom from all-cause mortality reported was 93.33% at 12-month. Mean aortic pressure gradient decreased from 52.71 ± 24.47 mmHg [with 0.89 ± 0.70 cm2 effective orifice area (EOA)] pre-operatively to 14.49 ± 6.58 mmHg (EOA: 1.85 ± 0.27 cm2) at 12-month. Overall, the mitral mean pressure gradient and EOA were 4.41 ± 1.69 mmHg and 2.67 ± 0.48 cm2, respectively, at 12-month. Significant improvement (P < 0.05) in the patients' quality of life was reported at all follow-ups.
Conclusions: The clinical safety and performance of the Dafodil pericardial bioprosthesis were favourable at 12-month. Moreover, a study with a larger patient population and longer follow-up is warranted to further assess the device.
Trial registration: Dafodil-1 trial has been prospectively registered on 10/07/2017 under Clinical Trial Registry-India (http://www.ctri.nic.in). (Registration number: CTRI/2017/07/009008).
Keywords: Aortic valve; Bioprosthesis; Heart valve prosthesis implantation; Hemodynamic performance; Mitral valve; Quality of life.
Conflict of interest statement
All the authors declare that they have no competing interests.
Figures




References
-
- Mannam G, Mishra Y, Modi R, Gokhale AGK, Sethuratnam R, Pandey K, et al. Early hemodynamic performance of the trifecta surgical bioprosthesis aortic valve in Indian patient population: 12 month outcomes of the EVEREST post-market study. J Cardiothorac Surg. 2018;13:96. doi: 10.1186/s13019-018-0783-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

