αvβ8 integrin adhesion and signaling pathways in development, physiology and disease
- PMID: 32540905
- PMCID: PMC7328133
- DOI: 10.1242/jcs.239434
αvβ8 integrin adhesion and signaling pathways in development, physiology and disease
Abstract
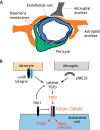
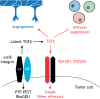
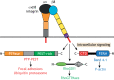
Cells must interpret a complex milieu of extracellular cues to modulate intracellular signaling events linked to proliferation, differentiation, migration and other cellular processes. Integrins are heterodimeric transmembrane proteins that link the extracellular matrix (ECM) to the cytoskeleton and control intracellular signaling events. A great deal is known about the structural and functional properties for most integrins; however, the adhesion and signaling pathways controlled by αvβ8 integrin, which was discovered nearly 30 years ago, have only recently been characterized. αvβ8 integrin is a receptor for ECM-bound forms of latent transforming growth factor β (TGFβ) proteins and promotes the activation of TGFβ signaling pathways. Studies of the brain, lung and immune system reveal that the αvβ8 integrin-TGFβ axis mediates cell-cell contact and communication within complex multicellular structures. Perturbing components of this axis results in aberrant cell-cell adhesion and signaling leading to the initiation of various pathologies, including neurodegeneration, fibrosis and cancer. As discussed in this Review, understanding the functions for αvβ8 integrin, its ECM ligands and intracellular effector proteins is not only an important topic in cell biology, but may lead to new therapeutic strategies to treat human pathologies related to integrin dysfunction.
Keywords: Angiogenesis; Cancer; Extracellular matrix; Microenvironment; Pathophysiology.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Integrins in cell adhesion and signaling.Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
-
Molecular Basis of the Ligand Binding Specificity of αvβ8 Integrin.J Biol Chem. 2016 May 27;291(22):11551-65. doi: 10.1074/jbc.M116.719138. Epub 2016 Mar 31. J Biol Chem. 2016. PMID: 27033701 Free PMC article.
-
Beta8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain.J Cell Sci. 2009 Jun 1;122(Pt 11):1842-51. doi: 10.1242/jcs.043257. J Cell Sci. 2009. PMID: 19461074 Free PMC article.
-
Inflammatory cues enhance TGFβ activation by distinct subsets of human intestinal dendritic cells via integrin αvβ8.Mucosal Immunol. 2017 May;10(3):624-634. doi: 10.1038/mi.2016.94. Epub 2016 Oct 26. Mucosal Immunol. 2017. PMID: 27782111 Free PMC article.
-
The role of integrins in TGFβ activation in the tumour stroma.Cell Tissue Res. 2016 Sep;365(3):657-73. doi: 10.1007/s00441-016-2474-y. Epub 2016 Aug 12. Cell Tissue Res. 2016. PMID: 27515461 Free PMC article. Review.
Cited by
-
Identification of AGR2 Gene-Specific Expression Patterns Associated with Epithelial-Mesenchymal Transition.Int J Mol Sci. 2022 Sep 16;23(18):10845. doi: 10.3390/ijms231810845. Int J Mol Sci. 2022. PMID: 36142758 Free PMC article.
-
Inhibition of integrin αvβ6 sparks T-cell antitumor response and enhances immune checkpoint blockade therapy in colorectal cancer.J Immunother Cancer. 2022 Feb;10(2):e003465. doi: 10.1136/jitc-2021-003465. J Immunother Cancer. 2022. PMID: 35131862 Free PMC article.
-
Human otic progenitor cell models of congenital hearing loss reveal potential pathophysiologic mechanisms of Zika virus and cytomegalovirus infections.mBio. 2024 Apr 10;15(4):e0019924. doi: 10.1128/mbio.00199-24. Epub 2024 Mar 5. mBio. 2024. PMID: 38440980 Free PMC article.
-
Model establishment and microarray analysis of mice with oxaliplatin‑induced hepatic sinusoidal obstruction syndrome.Mol Med Rep. 2022 Nov;26(5):346. doi: 10.3892/mmr.2022.12862. Epub 2022 Sep 30. Mol Med Rep. 2022. PMID: 36177905 Free PMC article.
-
Structural analysis of peptide binding to integrins for cancer detection and treatment.Biophys Rev. 2023 Jul 28;15(4):699-708. doi: 10.1007/s12551-023-01084-3. eCollection 2023 Aug. Biophys Rev. 2023. PMID: 37681100 Free PMC article. Review.
References
-
- Araya J., Cambier S., Markovics J. A., Wolters P., Jablons D., Hill A., Finkbeiner W., Jones K., Broaddus V. C., Sheppard D. et al. (2007). Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J. Clin. Invest. 117, 3551-3562. 10.1172/JCI32526 - DOI - PMC - PubMed
-
- Arnold T. D., Ferrero G. M., Qiu H., Phan I. T., Akhurst R. J., Huang E. J. and Reichardt L. F. (2012). Defective retinal vascular endothelial cell development as a consequence of impaired integrin αVβ8-mediated activation of transforming growth factor-β. J. Neurosci. 32, 1197-1206. 10.1523/JNEUROSCI.5648-11.2012 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

