Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options
- PMID: 32540959
- PMCID: PMC7300324
- DOI: 10.1124/pr.120.019539
Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options
Abstract
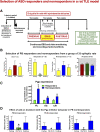
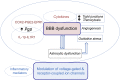
Epilepsy is a chronic neurologic disorder that affects over 70 million people worldwide. Despite the availability of over 20 antiseizure drugs (ASDs) for symptomatic treatment of epileptic seizures, about one-third of patients with epilepsy have seizures refractory to pharmacotherapy. Patients with such drug-resistant epilepsy (DRE) have increased risks of premature death, injuries, psychosocial dysfunction, and a reduced quality of life, so development of more effective therapies is an urgent clinical need. However, the various types of epilepsy and seizures and the complex temporal patterns of refractoriness complicate the issue. Furthermore, the underlying mechanisms of DRE are not fully understood, though recent work has begun to shape our understanding more clearly. Experimental models of DRE offer opportunities to discover, characterize, and challenge putative mechanisms of drug resistance. Furthermore, such preclinical models are important in developing therapies that may overcome drug resistance. Here, we will review the current understanding of the molecular, genetic, and structural mechanisms of ASD resistance and discuss how to overcome this problem. Encouragingly, better elucidation of the pathophysiological mechanisms underpinning epilepsies and drug resistance by concerted preclinical and clinical efforts have recently enabled a revised approach to the development of more promising therapies, including numerous potential etiology-specific drugs ("precision medicine") for severe pediatric (monogenetic) epilepsies and novel multitargeted ASDs for acquired partial epilepsies, suggesting that the long hoped-for breakthrough in therapy for as-yet ASD-resistant patients is a feasible goal. SIGNIFICANCE STATEMENT: Drug resistance provides a major challenge in epilepsy management. Here, we will review the current understanding of the molecular, genetic, and structural mechanisms of drug resistance in epilepsy and discuss how the problem might be overcome.
Copyright © 2020 by The Author(s).
Figures




References
-
- Abbott NJ. (2013) Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36:437–449. - PubMed
-
- Asadi-Pooya AA, Razavizadegan SM, Abdi-Ardekani A, Sperling MR. (2013) Adjunctive use of verapamil in patients with refractory temporal lobe epilepsy: a pilot study. Epilepsy Behav 29:150–154. - PubMed
-
- Avemary J, Salvamoser JD, Peraud A, Rémi J, Noachtar S, Fricker G, Potschka H. (2013) Dynamic regulation of P-glycoprotein in human brain capillaries. Mol Pharm 10:3333–3341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

