Differentiation of the human PAX7-positive myogenic precursors/satellite cell lineage in vitro
- PMID: 32541004
- PMCID: PMC7328153
- DOI: 10.1242/dev.187344
Differentiation of the human PAX7-positive myogenic precursors/satellite cell lineage in vitro
Abstract
Satellite cells (SC) are muscle stem cells that can regenerate adult muscles upon injury. Most SC originate from PAX7+ myogenic precursors set aside during development. Although myogenesis has been studied in mouse and chicken embryos, little is known about human muscle development. Here, we report the generation of human induced pluripotent stem cell (iPSC) reporter lines in which fluorescent proteins have been introduced into the PAX7 and MYOG loci. We use single cell RNA sequencing to analyze the developmental trajectory of the iPSC-derived PAX7+ myogenic precursors. We show that the PAX7+ cells generated in culture can produce myofibers and self-renew in vitro and in vivo Together, we demonstrate that cells exhibiting characteristics of human fetal satellite cells can be produced in vitro from iPSC, opening interesting avenues for muscular dystrophy cell therapy. This work provides significant insights into the development of the human myogenic lineage.
Keywords: Human development; PAX7; Pluripotent stem cell; Satellite cell; Skeletal muscle.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsOlivier Pourquie is a founder and shareholder of Anagenesis Biotechnologies.
Figures
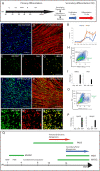

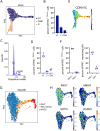
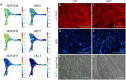

References
-
- Alexander M. S., Rozkalne A., Colletta A., Spinazzola J. M., Johnson S., Rahimov F., Meng H., Lawlor M. W., Estrella E., Kunkel L. M. et al. (2016). CD82 is a marker for prospective isolation of human muscle satellite cells and is linked to muscular dystrophies. Cell Stem Cell 19, 800-807. 10.1016/j.stem.2016.08.006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

