Stromal β-catenin activation impacts nephron progenitor differentiation in the developing kidney and may contribute to Wilms tumor
- PMID: 32541007
- PMCID: PMC7406317
- DOI: 10.1242/dev.189597
Stromal β-catenin activation impacts nephron progenitor differentiation in the developing kidney and may contribute to Wilms tumor
Abstract
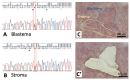
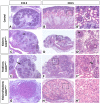
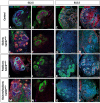
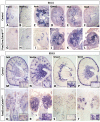
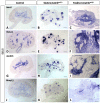
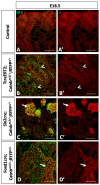
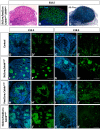
Wilms' tumor (WT) morphologically resembles the embryonic kidney, consisting of blastema, epithelial and stromal components, suggesting tumors arise from the dysregulation of normal development. β-Catenin activation is observed in a significant proportion of WTs; however, much remains to be understood about how it contributes to tumorigenesis. Although activating β-catenin mutations are observed in both blastema and stromal components of WT, current models assume that activation in the blastemal lineage is causal. Paradoxically, studies performed in mice suggest that activation of β-catenin in the nephrogenic lineage results in loss of nephron progenitor cell (NPC) renewal, a phenotype opposite to WT. Here, we show that activation of β-catenin in the stromal lineage non-autonomously prevents the differentiation of NPCs. Comparisons of the transcriptomes of kidneys expressing an activated allele of β-catenin in the stromal or nephron progenitor cells reveals that human WT more closely resembles the stromal-lineage mutants. These findings suggest that stromal β-catenin activation results in histological and molecular features of human WT, providing insights into how alterations in the stromal microenvironment may play an active role in tumorigenesis.
Keywords: Renal development; Renal interstitium; Stroma; Wilms' tumor; β-Catenin.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Bremnes R. M., Dønnem T., Al-Saad S., Al-Shibli K., Andersen S., Sirera R., Camps C., Marinez I. and Busund L.-T. (2011). The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 6, 209-217. 10.1097/JTO.0b013e3181f8a1bd - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

