Probing and manipulating embryogenesis via nanoscale thermometry and temperature control
- PMID: 32541064
- PMCID: PMC7334529
- DOI: 10.1073/pnas.1922730117
Probing and manipulating embryogenesis via nanoscale thermometry and temperature control
Abstract
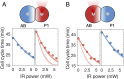
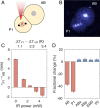
Understanding the coordination of cell-division timing is one of the outstanding questions in the field of developmental biology. One active control parameter of the cell-cycle duration is temperature, as it can accelerate or decelerate the rate of biochemical reactions. However, controlled experiments at the cellular scale are challenging, due to the limited availability of biocompatible temperature sensors, as well as the lack of practical methods to systematically control local temperatures and cellular dynamics. Here, we demonstrate a method to probe and control the cell-division timing in Caenorhabditis elegans embryos using a combination of local laser heating and nanoscale thermometry. Local infrared laser illumination produces a temperature gradient across the embryo, which is precisely measured by in vivo nanoscale thermometry using quantum defects in nanodiamonds. These techniques enable selective, controlled acceleration of the cell divisions, even enabling an inversion of division order at the two-cell stage. Our data suggest that the cell-cycle timing asynchrony of the early embryonic development in C. elegans is determined independently by individual cells rather than via cell-to-cell communication. Our method can be used to control the development of multicellular organisms and to provide insights into the regulation of cell-division timings as a consequence of local perturbations.
Keywords: cell-cycle control; cell-division asymmetry; nanoscale thermometry; nitrogen-vacancy centers; quantum sensing.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Gönczy P., Mechanisms of asymmetric cell division: Flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355–366 (2008). - PubMed
-
- Budirahardja Y., Gönczy P., PLK-1 asymmetry contributes to asynchronous cell division of C. elegans embryos. Development 135, 1303–1313 (2008). - PubMed
-
- Tavernier N., Labbé J., Pintard L., Cell cycle timing regulation during asynchronous divisions of the early C. elegans embryo. Exp. Cell Res. 337, 243–248 (2015). - PubMed
-
- Brauchle M., Baumer K., Gönczy P., Differential activation of the DNA replication checkpoint contributes to asynchrony of cell division in C. elegans embryos. Curr. Biol. 13, 819–827 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

