Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer
- PMID: 32541132
- PMCID: PMC7885710
- DOI: 10.4103/2045-9912.285560
Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer
Abstract
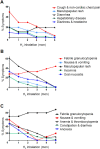
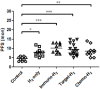
Chemotherapy, targeted therapy, and immunotherapy are used against advanced non-small cell lung cancer. A clinically efficacious method for relieving the adverse events associated of such therapies is lacking. Fifty-eight adult patients were enrolled in our trial to relieve pulmonary symptoms or the adverse events of drugs. Twenty patients who refused drug treatment were assigned equally and randomly to a hydrogen (H2)-only group and a control group. According to the results of tumor-gene mutations and drug-sensitivity tests, 10, 18, and 10 patients were enrolled into chemotherapy, targeted therapy, and immunotherapy groups in which these therapies were combined with H2-therapy, respectively. Patients underwent H2 inhalation for 4-5 hours per day for 5 months or stopped when cancer recurrence. Before study initiation, the demographics (except for tumor-mutation genes) and pulmonary symptoms (except for moderate cough) of the five groups showed no significant difference. During the first 5 months of treatment, the prevalence of symptoms of the control group increased gradually, whereas that of the four treatment groups decreased gradually. After 16 months of follow-up, progression-free survival of the control group was lower than that of the H2-only group, and significantly lower than that of H2 + chemotherapy, H2 + targeted therapy, and H2 + immunotherapy groups. In the combined-therapy groups, most drug-associated adverse events decreased gradually or even disappeared. H2 inhalation was first discovered in the clinic that can be used to control tumor progression and alleviate the adverse events of medications for patients with advanced non-small cell lung cancer. This study was approved by the Ethics Committee of Fuda Cancer Hospital of Jinan University on December 7, 2018 (approval No. Fuda20181207), and was registered at ClinicalTrials.gov (Identifier: NCT03818347) on January 28, 2019.
Keywords: NSCLC; PFS; adverse event; chemotherapy; hydrogen; immunotherapy; non-small-cell lung cancer; progression-free survival; targeted drug.
Conflict of interest statement
None declared.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
-
- de Groot PM, Chung JH, Ackman JB, et al. ACR Appropriateness Criteria® noninvasive clinical staging of primary lung cancer. J Am Coll Radiol. 2019;16:S184–S195. - PubMed
-
- Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16:653–660. - PubMed
-
- Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC) Respirology. 2015;20:370–378. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

