New Surgical Circulatory Support System Outcomes
- PMID: 32541335
- PMCID: PMC7316144
- DOI: 10.1097/MAT.0000000000001194
New Surgical Circulatory Support System Outcomes
Abstract
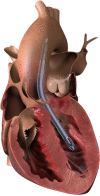
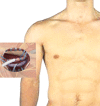
We report the first U.S. experience of the recently approved micro-axial surgical heart pump for the treatment of ongoing cardiogenic shock following acute myocardial infarction (AMICGS), postcardiotomy cardiogenic shock (PCCS), cardiomyopathy including myocarditis, high-risk percutaneous coronary intervention (HRPCI), and coronary artery bypass surgery (HRCABG). Demographic, procedural, hemodynamic, and outcome data were obtained from the manufacturer's quality database of all Impella 5.5 implants at three centers. Fifty-five patients underwent an Impella 5.5 implant for cardiomyopathy (45%), AMICGS (29%), PCCS (13%), preop CABG (5%), OPCAB (4%), and other (4%). Thirty-five patients (63.6%) were successfully weaned off device with recovery of native heart function. Eleven patients (20.0%) were bridged to another therapy, two patients (3.6%) expired while on support, and in seven patients (12.7%) care was withdrawn. Overall survival was 83.6%. There were no device-related strokes, hemolysis, or limb ischemia observed. Four patients experienced purge sidearm damage, resulting in a pump stop in two patients. The new micro-axial surgical heart pump demonstrated successful clinical and device performance in providing both full hemodynamic support and ventricular unloading for patients with AMICGS, decompensated cardiomyopathy, and high-risk cardiac procedures. In this early U.S. experience, 83.6% of patients survived to explant with 76.1% of these patients recovering native heart function.
Conflict of interest statement
Disclosure: D.R., E.S., and M.A. are consultants, speakers, and members of the surgical advisory board for Abiomed, Inc. The authors have no conflicts of interest to report.
Figures



References
-
- Rihal CS, Naidu SS, Givertz MM, et al. ; Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC): 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 2015; 65:e7–e26. - PubMed
-
- Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: Incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014; 64:1407–1415. - PubMed
-
- O’Neill WW, Grines C, Schreiber T, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J 2018; 202:33–38. - PubMed
-
- Pahuja M, Adegbala O, Mishra T, et al. Trends in the incidence of in-hospital mortality, cardiogenic shock, and utilization of mechanical circulatory support devices in myocarditis (Analysis of National Inpatient Sample Data, 2005-2014). J Card Fail 2019; 25:457–467. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

