Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptide-dependent hyperalgesic priming to a migraine trigger
- PMID: 32541386
- PMCID: PMC7572536
- DOI: 10.1097/j.pain.0000000000001953
Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptide-dependent hyperalgesic priming to a migraine trigger
Abstract
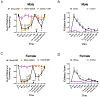
Migraine is one of the most disabling disorders worldwide but the underlying mechanisms are poorly understood. Stress is consistently reported as a common trigger of migraine attacks. Here, we show that repeated stress in mice causes migraine-like behaviors that are responsive to a migraine therapeutic. Adult female and male mice were exposed to 2 hours of restraint stress for 3 consecutive days, after which they demonstrated facial mechanical hypersensitivity and facial grimace responses that were resolved by 14 days after stress. Hypersensitivity or grimace was not observed in either control animals or those stressed for only 1 day. After return to baseline, the nitric oxide donor sodium nitroprusside (SNP; 0.1 mg/kg) elicited mechanical hypersensitivity in stressed but not in control animals, demonstrating the presence of hyperalgesic priming. This suggests the presence of a migraine-like state, because nitric oxide donors are reliable triggers of attacks in migraine patients but not controls. The stress paradigm also caused priming responses to dural pH 7.0 treatment. The presence of this primed state after stress is not permanent because it was no longer present at 35 days after stress. Finally, mice received either the calcitonin gene-related peptide monoclonal antibody ALD405 (10 mg/kg) 24 hours before SNP or a coinjection of sumatriptan (0.6 mg/kg). ALD405, but not sumatriptan, blocked the facial hypersensitivity due to SNP. This stress paradigm in mice and the subsequent primed state caused by stress allow further preclinical investigation of mechanisms contributing to migraine, particularly those caused by common triggers of attacks.
Figures
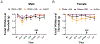
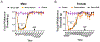
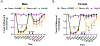

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

