Dose-response of rPMS for upper Limb hemiparesis after stroke
- PMID: 32541528
- PMCID: PMC7302622
- DOI: 10.1097/MD.0000000000020752
Dose-response of rPMS for upper Limb hemiparesis after stroke
Abstract
Introduction: Repetitive peripheral magnetic stimulation (rPMS) therapy is an innovative and minimally invasive neurorehabilitative technique and has been shown to facilitate neural plasticity. However, there is at present no research that clarifies the dose-response of rPMS therapy on the recovery of upper limb hemiparesis after stroke. This trial aims to clarify the dose-response of rPMS therapy combined with intensive occupational therapy (OT) for chronic stroke patients with moderate to severe upper limb hemiparesis.
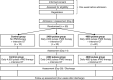
Methods and analysis: This multicenter, prospective, assessor-blinded, randomized controlled study with 3 parallel groups will be conducted from January 20, 2020 to September 30, 2022. Fifty patients will be randomly assigned in a ratio of 1:2:2 to the control group, the group receiving daily 2400 pulses of rPMS, or the group receiving daily 4800 pulses of rPMS, respectively. From the day after admission (Day 1), rPMS therapy and intensive OT will be initiated. The primary outcome is the change in the motor function of the affected upper extremity (Fugl-Meyer Assessment) between the time of admission (Day 0) and the day after 2 weeks of treatment (Day 14). Secondary outcomes will include the changes in spasticity, active range of motion, motor evoked potential, and activity of daily living.
Ethics and dissemination: The study was approved by the Jikei University Certified Review Board for all institutions (reference number: JKI19-020). Results of the primary and secondary outcomes will be published in a peer-reviewed journal and presented at international congresses. The results will also be disseminated to patients.
Trial registration number: jRCTs032190191.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Nichols-Larsen D, Clark P, Zeringue A, et al. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke 2005;36:1480–4. - PubMed
-
- Howlett O, Lannin N, Ada L, et al. Functional electrical stimulation improves activity after stroke: a systematic review with meta-analysis. Arch Phys Med Rehabil 2015;96:934–43. - PubMed
-
- Yang J, Liao C, Huang S, et al. Effectiveness of electrical stimulation therapy in improving arm function after stroke: a systematic review and a meta-analysis of randomised controlled trials. Clin Rehabil 2019;33:1286–97. - PubMed
-
- Abo M, Kakuda W, Momosaki R, et al. Randomized, multicenter, comparative study of NEURO versus CIMT in poststroke patients with upper limb hemiparesis: The NEURO-VERIFY Study. Int J Stroke 2014;9:607–12. - PubMed
-
- Lefaucheur J, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150–206. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical