AAV-mediated FOXG1 gene editing in human Rett primary cells
- PMID: 32541681
- PMCID: PMC7608362
- DOI: 10.1038/s41431-020-0652-6
AAV-mediated FOXG1 gene editing in human Rett primary cells
Abstract
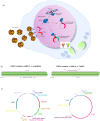
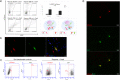
Variations in the Forkhead Box G1 (FOXG1) gene cause FOXG1 syndrome spectrum, including the congenital variant of Rett syndrome, characterized by early onset of regression, Rett-like and jerky movements, and cortical visual impairment. Due to the largely unknown pathophysiological mechanisms downstream the impairment of this transcriptional regulator, a specific treatment is not yet available. Since both haploinsufficiency and hyper-expression of FOXG1 cause diseases in humans, we reasoned that adding a gene under nonnative regulatory sequences would be a risky strategy as opposed to a genome editing approach where the mutated gene is reversed into wild-type. Here, we demonstrate that an adeno-associated viruses (AAVs)-coupled CRISPR/Cas9 system is able to target and correct FOXG1 variants in patient-derived fibroblasts, induced Pluripotent Stem Cells (iPSCs) and iPSC-derived neurons. Variant-specific single-guide RNAs (sgRNAs) and donor DNAs have been selected and cloned together with a mCherry/EGFP reporter system. Specific sgRNA recognition sequences were inserted upstream and downstream Cas9 CDS to allow self-cleavage and inactivation. We demonstrated that AAV serotypes vary in transduction efficiency depending on the target cell type, the best being AAV9 in fibroblasts and iPSC-derived neurons, and AAV2 in iPSCs. Next-generation sequencing (NGS) of mCherry+/EGFP+ transfected cells demonstrated that the mutated alleles were repaired with high efficiency (20-35% reversion) and precision both in terms of allelic discrimination and off-target activity. The genome editing strategy tested in this study has proven to precisely repair FOXG1 and delivery through an AAV9-based system represents a step forward toward the development of a therapy for Rett syndrome.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Papa FT, Mencarelli MA, Caselli R, Katzaki E, Sampieri K, Meloni I, et al. A 3 Mb deletion in 14q12 causes severe mental retardation, mild facial dysmorphisms and Rett-like features. Am J Med Genet A. 2008;146A:1994–8. - PubMed
-
- Rolando S. Rett syndrome: report of eight cases. Brain Dev. 1985;7:290–6. - PubMed
-
- Yeung A, Bruno D, Scheffer IE, Carranza D, Burgess T, Slater HR, et al. 4.45 Mb microduplication in chromosome band 14q12 including FOXG1 in a girl with refractory epilepsy and intellectual impairment. Eur J Med Genet. 2009;52:440–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

