Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities
- PMID: 32541893
- PMCID: PMC7326705
- DOI: 10.1038/s41582-020-0362-2
Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities
Abstract
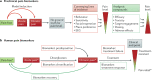
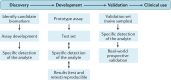
Pain medication plays an important role in the treatment of acute and chronic pain conditions, but some drugs, opioids in particular, have been overprescribed or prescribed without adequate safeguards, leading to an alarming rise in medication-related overdose deaths. The NIH Helping to End Addiction Long-term (HEAL) Initiative is a trans-agency effort to provide scientific solutions to stem the opioid crisis. One component of the initiative is to support biomarker discovery and rigorous validation in collaboration with industry leaders to accelerate high-quality clinical research into neurotherapeutics and pain. The use of objective biomarkers and clinical trial end points throughout the drug discovery and development process is crucial to help define pathophysiological subsets of pain, evaluate target engagement of new drugs and predict the analgesic efficacy of new drugs. In 2018, the NIH-led Discovery and Validation of Biomarkers to Develop Non-Addictive Therapeutics for Pain workshop convened scientific leaders from academia, industry, government and patient advocacy groups to discuss progress, challenges, gaps and ideas to facilitate the development of biomarkers and end points for pain. The outcomes of this workshop are outlined in this Consensus Statement.
Conflict of interest statement
S.I., M.J.I. and M.A.P. are NIH employees. A.H.A. is an employee of Teva Pharmaceuticals. D.B. consults for Biogen. A.B. is the Chief Science Officer of Mycroft Bioanalytics, a precision medicine company focused on pain. C.Y.S. is Chief Scientific Officer at neurotecnix, a start-up that develops EEG-based pain biomarkers; a consultant for Asahi Kasei Pharmaceuticals, Japan and PainQX, USA; has received remuneration for symposia funded by the International Association for the Study of Pain, Medtronic, Boston Scientific and NIH/NINDS; and is an inventor on US patents 61/328,583, 9,486,632, 62/203,798 and 62/329,345, all of which relate to the detection or treatment of pain. C.N.S. has received funding from Merck, Helixmith and the Utley Foundation; has acted as a consultant for Lilly Research Laboratories, Covance, Genentech, Alkermes, Arena, Nevaker and Heron Therapeutics; is an inventor on European patent 01942162, US patent 8,309,507 and CA patent 2,499,987, all of which relate to the treatment of central neuropathic pain; and has non-financial competing interests as Director of Rick Hansen Institute, Chairman of Medical and Scientific Advisory Committee, United Spinal Association and membership on the Interagency Pain Research Coordinating Committee (NIH/HHS). J.S. is an employee and stock owner with Biogen. T.D.W. is an inventor on US patent US 2018/0055407. A.D.W. is a consultant for Analgesic Solutions and Pfizer, and has received an Investigator-Initiated Grant from Collegium Pharmaceuticals. The other authors declare no competing interests.
Figures
References
-
- Treede RD, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11) Pain. 2019;160:19–27. - PubMed
-
- Von Korff M, et al. United States National Pain Strategy for population research: concepts, definitions, and pilot data. J. Pain. 2016;17:1068–1080. - PubMed
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. - PMC - PubMed
-
- Nahin RL, Sayer B, Stussman BJ, Feinberg TM. Eighteen-year trends in the prevalence of, and health care use for, noncancer pain in the United States: data from the Medical Expenditure Panel Survey. J. Pain. 2019;20:796–809. - PubMed
-
- US Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (National Academies, 2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

