Development of a small animal model for deer tick virus pathogenesis mimicking human clinical outcome
- PMID: 32542017
- PMCID: PMC7316340
- DOI: 10.1371/journal.pntd.0008359
Development of a small animal model for deer tick virus pathogenesis mimicking human clinical outcome
Abstract
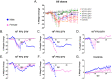
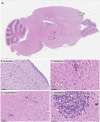
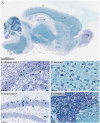
Powassan virus (POWV) is a tick-borne flavivirus that encompasses two genetic lineages, POWV (Lineage I) and deer tick virus (DTV, Lineage II). In recent years, the incidence of reported POWV disease cases has increased, coupled with an expanded geographic range of the DTV tick vector, Ixodes scapularis. POWV and DTV are serologically indistinguishable, and it is not known whether clinical manifestations, pathology, or disease outcome differ between the two viruses. Six-week-old male and female BALB/c mice were footpad-inoculated with DTV doses ranging from 101 to 105 FFU. Dose-independent mortality, morbidity, and organ viral loads were observed for mice inoculated with sequentially increasing doses of DTV. By study completion, all surviving mice had cleared their viremias but detectable levels of negative-sense DTV RNA were present in the brain, indicating viral persistence of infectious DTV in the central nervous system. For mice that succumbed to disease, neuropathology revealed meningoencephalitis characterized by microscopic lesions with widespread distribution of viral RNA in the brain. These findings, coupled with the rapid onset of neurological signs of disease and high viral titers in nervous tissue, highlight the neurotropism of DTV in this mouse model. Additionally, disease outcome for DTV-infected mice was not affected by sex, as males and females were equally susceptible to disease. This is the first study to comprehensively characterize the clinical disease outcome in a small animal model across a spectrum of POWV/DTV infection doses. Here, we developed a small animal model for DTV pathogenesis that mimics the manifestations of POWV disease in humans. Since it is currently not known whether DTV and POWV differ in their capacity to cause human disease, the animal model detailed in our study could be utilized in future comparative pathogenesis studies, or as a platform for testing the efficacy of vaccines, and anti-virals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Tick Saliva Enhances Powassan Virus Transmission to the Host, Influencing Its Dissemination and the Course of Disease.J Virol. 2015 Aug;89(15):7852-60. doi: 10.1128/JVI.01056-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995246 Free PMC article.
-
Isolation of deer tick virus (Powassan virus, lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State.Parasit Vectors. 2013 Jul 15;6:185. doi: 10.1186/1756-3305-6-185. Parasit Vectors. 2013. PMID: 24016533 Free PMC article.
-
Age-dependent Powassan virus lethality is linked to glial cell activation and divergent neuroinflammatory cytokine responses in a murine model.J Virol. 2024 Aug 20;98(8):e0056024. doi: 10.1128/jvi.00560-24. Epub 2024 Aug 1. J Virol. 2024. PMID: 39087762 Free PMC article.
-
Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America.Vector Borne Zoonotic Dis. 2017 Jul;17(7):453-462. doi: 10.1089/vbz.2017.2110. Epub 2017 May 12. Vector Borne Zoonotic Dis. 2017. PMID: 28498740 Free PMC article. Review.
-
The emergence of human Powassan virus infection in North America.Ticks Tick Borne Dis. 2020 Nov;11(6):101540. doi: 10.1016/j.ttbdis.2020.101540. Epub 2020 Aug 7. Ticks Tick Borne Dis. 2020. PMID: 32993949 Review.
Cited by
-
Powassan Virus Induces Structural Changes in Human Neuronal Cells In Vitro and Murine Neurons In Vivo.Pathogens. 2022 Oct 21;11(10):1218. doi: 10.3390/pathogens11101218. Pathogens. 2022. PMID: 36297275 Free PMC article.
-
Differential Virulence and Host-Specific Fitness of Regionally Distinct Human-Derived Powassan Virus Lineage 2 Strains.Am J Trop Med Hyg. 2025 May 13;113(1):106-116. doi: 10.4269/ajtmh.24-0776. Print 2025 Jul 2. Am J Trop Med Hyg. 2025. PMID: 40359924 Free PMC article.
-
Horizontal and Vertical Transmission of Powassan Virus by the Invasive Asian Longhorned Tick, Haemaphysalis longicornis, Under Laboratory Conditions.Front Cell Infect Microbiol. 2022 Jul 1;12:923914. doi: 10.3389/fcimb.2022.923914. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35846754 Free PMC article.
-
Pathogenicity and virulence of Powassan virus.Virulence. 2025 Dec;16(1):2523887. doi: 10.1080/21505594.2025.2523887. Epub 2025 Jun 26. Virulence. 2025. PMID: 40545598 Free PMC article. Review.
-
Of Murines and Humans: Modeling Persistent Powassan Disease in C57BL/6 Mice.mBio. 2023 Apr 25;14(2):e0360622. doi: 10.1128/mbio.03606-22. Epub 2023 Feb 21. mBio. 2023. PMID: 36809119 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

