Format and geometries matter: Structure-based design defines the functionality of bispecific antibodies
- PMID: 32542108
- PMCID: PMC7283971
- DOI: 10.1016/j.csbj.2020.05.006
Format and geometries matter: Structure-based design defines the functionality of bispecific antibodies
Abstract
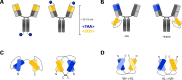
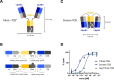
Multispecific antibodies can be generated in different formats. More than two decades of R&D in the field of bispecific antibody engineering revealed that the design and choice of format can have a profound impact on the antibody functionality. This holds in particular true for entities that elicit (inter-)cellular processes such as receptor activation, receptor internalization, receptor clustering or the formation of immunological synapses between two cells. This review covers design parameters that influence the functionality of multispecific formats, with particular focus on T cell-recruiting bispecific antibodies. We describe formats that display the same size and domain sequences but a varying geometry. The structural composition of (artificial) immune synapses is reviewed and allows conclusions why some formats that share size and domain composition are more effective than others. To support the statement that the geometry matters, we present a recently designed antibody format that is characterized by its compact shape. The TriFab-Contorsbody consists of two tumor cell-targeting entities and one moiety for T cell recruitment. The unique barrel-like shape provides a 35-fold increase in potency compared to an IgG-like molecule with identical domain sequences.
Keywords: Antibody format; Geometry; Immune synapse; Protein engineering; T cell bispecific.
© 2020 The Author(s).
Figures


References
-
- Asano R., Kumagai T., Nagai K., Taki S., Shimomura I., Arai K. Domain order of a bispecific diabody dramatically enhances its antitumor activity beyond structural format conversion: the case of the hEx3 diabody. Protein Eng Des Sel. 2013;26:359–367. - PubMed
-
- Bacac Marina, Colombetti Sara, Herter Sylvia, Sam Johannes, Perro Mario, Chen Stanford. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24:4785–4797. - PubMed
-
- Bargou R., Leo E., Zugmaier G., Klinger M., Goebeler M., Knop S. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
