Developments in Photoredox/Nickel Dual-Catalyzed 1,2-Difunctionalizations
- PMID: 32542207
- PMCID: PMC7295134
- DOI: 10.1016/j.chempr.2020.05.013
Developments in Photoredox/Nickel Dual-Catalyzed 1,2-Difunctionalizations
Abstract
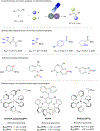
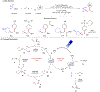
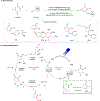
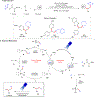
Progress in Ni/photoredox dual catalysis has enabled the construction of C(sp3)-hybridized centers under extremely mild reaction conditions in the presence of diverse functional groups. These strategies, however, are mainly restricted to the assembly of one C-C or C-heteroatom linkage because of the competitive two-component reactions and facile β-hydride elimination from alkylmetal complexes. Recently, photoinduced nickel-catalyzed 1,2-difunctionalizations of alkenes and alkynes have attracted extensive research efforts as they allow the construction of two sequential chemical bonds from inexpensive starting materials in one pot. Herein, we explore recent advances, state the current challenges, and discuss perspectives on the design of new catalytic systems.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
