Glucagon-like peptide 2 for intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans
- PMID: 32542357
- PMCID: PMC7498363
- DOI: 10.1182/blood.2020005957
Glucagon-like peptide 2 for intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans
Abstract
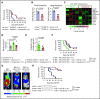
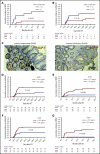
Acute graft-versus-host disease (GVHD) is a life-threatening complication after allogeneic hematopoietic cell transplantation (allo-HCT). Although currently used GVHD treatment regimens target the donor immune system, we explored here an approach that aims at protecting and regenerating Paneth cells (PCs) and intestinal stem cells (ISCs). Glucagon-like-peptide-2 (GLP-2) is an enteroendocrine tissue hormone produced by intestinal L cells. We observed that acute GVHD reduced intestinal GLP-2 levels in mice and patients developing GVHD. Treatment with the GLP-2 agonist, teduglutide, reduced de novo acute GVHD and steroid-refractory GVHD, without compromising graft-versus-leukemia (GVL) effects in multiple mouse models. Mechanistically GLP-2 substitution promoted regeneration of PCs and ISCs, which enhanced production of antimicrobial peptides and caused microbiome changes. GLP-2 expanded intestinal organoids and reduced expression of apoptosis-related genes. Low numbers of L cells in intestinal biopsies and high serum levels of GLP-2 were associated with a higher incidence of nonrelapse mortality in patients undergoing allo-HCT. Our findings indicate that L cells are a target of GVHD and that GLP-2-based treatment of acute GVHD restores intestinal homeostasis via an increase of ISCs and PCs without impairing GVL effects. Teduglutide could become a novel combination partner for immunosuppressive GVHD therapy to be tested in clinical trials.
Conflict of interest statement
Conflict-of-interest disclosure: B.R.B. receives remuneration as an advisor to Kamon Pharmaceuticals, Inc, Five Prime Therapeutics Inc, Regeneron Pharmaceuticals, Magenta Therapeutics, and BlueRock Therapeutics; receives research support from Fate Therapeutics, RXi Pharmaceuticals, Alpine Immune Sciences, Inc, AbbVie Inc, BlueRock Therapeutics, the Leukemia & Lymphoma Society, the Children’s Cancer Research Fund, and the KidsFirst Fund; and is a cofounder of Tmunity. The remaining authors declare no competing financial interests.
Figures






Comment in
-
I have a gut feeling….Blood. 2020 Sep 17;136(12):1380. doi: 10.1182/blood.2020007186. Blood. 2020. PMID: 32941633 No abstract available.
References
-
- Zeiser R, von Bubnoff N, Butler J, et al. ; REACH2 Trial Group . Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800-1810. - PubMed
-
- Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561-583. - PubMed
-
- Connor EE, Evock-Clover CM, Walker MP, Elsasser TH, Kahl S. Comparative Gut Physiology Symposium: Comparative physiology of glucagon-like peptide-2: Implications and applications for production and health of ruminants. J Anim Sci. 2015;93(2):492-501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

