Dosing Recommendations for Pediatric Patients With Renal Impairment
- PMID: 32542790
- PMCID: PMC8670561
- DOI: 10.1002/jcph.1676
Dosing Recommendations for Pediatric Patients With Renal Impairment
Abstract
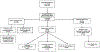
A treatment gap exists for pediatric patients with renal impairment. Alterations in renal clearance and metabolism of drugs render standard dosage regimens inappropriate and may lead to drug toxicity, but these studies are not routinely conducted during drug development. The objective of this study was to examine the clinical evidence behind current renal impairment dosage recommendations for pediatric patients in a standard pediatric dosing handbook. The sources of recommendations and comparisons included the pediatric dosing handbook (Lexicomp), the U.S. Food and Drug Administration-approved manufacturer's labels, and published studies in the literature. One hundred twenty-six drugs in Lexicomp had pediatric renal dosing recommendations. Only 14% (18 of 126) of Lexicomp pediatric renal dosing recommendations referenced a pediatric clinical study, and 15% of manufacturer's labels (19 of 126) described specific dosing regimens for renally impaired pediatric patients. Forty-two products had published information on pediatric renal dosing, but 19 (45%) were case studies. When pediatric clinical studies were not referenced in Lexicomp, the renal dosing recommendations followed the adult and pediatric dosing recommendations on the manufacturer's label. Clinical evidence in pediatric patients does not exist for most renal dosing recommendations in a widely used pediatric dosing handbook, and the adult renal dosing recommendations from the manufacturer's label are currently the primary source of pediatric renal dosing information.
Keywords: MIDD (model-informed drug development); drug development; pediatrics (PED); renal disease; special populations.
© 2020, The American College of Clinical Pharmacology.
Conflict of interest statement
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- US Food and Drug Administration: New Pediatric Labeling Information Database. 2019. https://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingd.... Accessed May 14, 2020.
-
- Shirkey H Therapeutic orphans. J Pediatr. 1968;72(1):119–120. - PubMed
-
- Group KKDIGOCW. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

