Development of an automatic integrated gene detection system for novel severe acute respiratory syndrome-related coronavirus (SARS-CoV2)
- PMID: 32543298
- PMCID: PMC7473122
- DOI: 10.1080/22221751.2020.1782774
Development of an automatic integrated gene detection system for novel severe acute respiratory syndrome-related coronavirus (SARS-CoV2)
Abstract
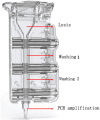
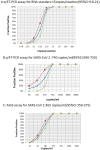
In December 2019, Wuhan, China suffered a serious outbreak of a novel coronavirus infectious disease (COVID) caused by novel severe acute respiratory syndrome-related coronavirus (SARS-CoV 2). To quickly identify the pathogen, we designed and screened primer sets, and established a sensitive and specific qRT-PCR assay for SARS-CoV 2; the lower limit of detection (LOD) was 14.8 (95% CI: 9.8-21) copies per reaction. We combined this qRT-PCR assay with an automatic integration system for nucleic acid extraction and amplification, thereby establishing an automatic integrated gene detection system (AIGS) for SARS-CoV 2. Cross reactive analysis performed in 20 other respiratory viruses and 37 nasopharyngeal swabs confirmed a 100% specificity of the assay. Using two fold diluted SARS-CoV 2 culture, the LOD of AIGS was confirmed to be 365 copies/ml (95% CI: 351-375), which was Comparable to that of conventional q RT-PCR (740 copies/ml, 95% CI: 689-750). Clinical performances of AIGS assay were assessed in 266 suspected COVID-19 clinical respiratory tract samples tested in parallel with a commercial kit. The clinical sensitivity of the AIGS test was 97.62% (95% CI: 0.9320-0.9951) based on the commercial kit test result, and concordance analysis showed a high agreement in SARS-CoV-2 detection between the two assays, Pearson R was 0.9623 (95% CI: 0.9523-0.9703). The results indicated that this AIGS could be used for rapid detection of SARS-CoV 2. With the advantage of simple operation and less time consuming, AIGS could be suitable for SARS-CoV2 detection in primary medical institutions, thus would do a great help to improve detection efficiency and control the spread of COVID-19.
Keywords: COVID-19; SARS-CoV2; automatic integrated gene detection system; qRT-PCR; rapid detection.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
Similar articles
-
A reverse-transcription recombinase-aided amplification assay for the rapid detection of N gene of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2).Virology. 2020 Oct;549:1-4. doi: 10.1016/j.virol.2020.07.006. Epub 2020 Jul 29. Virology. 2020. PMID: 32758712 Free PMC article.
-
Multiplexing primer/probe sets for detection of SARS-CoV-2 by qRT-PCR.J Clin Virol. 2020 Aug;129:104499. doi: 10.1016/j.jcv.2020.104499. Epub 2020 Jun 8. J Clin Virol. 2020. PMID: 32535397 Free PMC article.
-
Double-quencher probes improve detection sensitivity toward Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a reverse-transcription polymerase chain reaction (RT-PCR) assay.J Virol Methods. 2020 Oct;284:113926. doi: 10.1016/j.jviromet.2020.113926. Epub 2020 Jul 7. J Virol Methods. 2020. PMID: 32650037 Free PMC article.
-
Primer design for quantitative real-time PCR for the emerging Coronavirus SARS-CoV-2.Theranostics. 2020 Jun 1;10(16):7150-7162. doi: 10.7150/thno.47649. eCollection 2020. Theranostics. 2020. PMID: 32641984 Free PMC article. Review.
-
Laboratory diagnosis of SARS-CoV-2 - A review of current methods.J Infect Public Health. 2020 Jul;13(7):901-905. doi: 10.1016/j.jiph.2020.06.005. Epub 2020 Jun 7. J Infect Public Health. 2020. PMID: 32534946 Free PMC article. Review.
Cited by
-
Preparation of the luciferase-labeled antibody for improving the detection sensitivity of viral antigen.Virol J. 2022 Jul 28;19(1):126. doi: 10.1186/s12985-022-01855-6. Virol J. 2022. PMID: 35902865 Free PMC article.
-
Current methods and prospects of coronavirus detection.Talanta. 2021 Apr 1;225:121977. doi: 10.1016/j.talanta.2020.121977. Epub 2020 Dec 31. Talanta. 2021. PMID: 33592725 Free PMC article. Review.
-
COVID-19 Diagnostic Strategies. Part I: Nucleic Acid-Based Technologies.Bioengineering (Basel). 2021 Apr 17;8(4):49. doi: 10.3390/bioengineering8040049. Bioengineering (Basel). 2021. PMID: 33920513 Free PMC article. Review.
-
Performance evaluation of Biofire Film Array Respiratory Panel 2.1 for SARS-CoV-2 detection in a pediatric hospital setting.PLoS One. 2023 Oct 5;18(10):e0292314. doi: 10.1371/journal.pone.0292314. eCollection 2023. PLoS One. 2023. PMID: 37797063 Free PMC article.
-
Development of a quantitative one-step multiplex RT-qPCR assay for the detection of SARS-CoV-2 in a biological matrix.Int J Infect Dis. 2021 Mar;104:373-378. doi: 10.1016/j.ijid.2021.01.001. Epub 2021 Jan 9. Int J Infect Dis. 2021. PMID: 33434663 Free PMC article.
References
-
- Gorbalenya AE, Baker SC, Baric RS, et al. . The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020 Mar 2. [Epub ahead of print] Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. doi: 10.1038/s41564-020-0695-z - DOI - PMC - PubMed
-
- Meo SA, Alhowikan AM, Al-Khlaiwi T, et al. . Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020 Feb;24(4):2012–2019. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
