Effect of Continuous Glucose Monitoring on Glycemic Control in Adolescents and Young Adults With Type 1 Diabetes: A Randomized Clinical Trial
- PMID: 32543683
- PMCID: PMC7298603
- DOI: 10.1001/jama.2020.6940
Effect of Continuous Glucose Monitoring on Glycemic Control in Adolescents and Young Adults With Type 1 Diabetes: A Randomized Clinical Trial
Abstract
Importance: Adolescents and young adults with type 1 diabetes exhibit the worst glycemic control among individuals with type 1 diabetes across the lifespan. Although continuous glucose monitoring (CGM) has been shown to improve glycemic control in adults, its benefit in adolescents and young adults has not been demonstrated.
Objective: To determine the effect of CGM on glycemic control in adolescents and young adults with type 1 diabetes.
Design, setting, and participants: Randomized clinical trial conducted between January 2018 and May 2019 at 14 endocrinology practices in the US including 153 individuals aged 14 to 24 years with type 1 diabetes and screening hemoglobin A1c (HbA1c) of 7.5% to 10.9%.
Interventions: Participants were randomized 1:1 to undergo CGM (CGM group; n = 74) or usual care using a blood glucose meter for glucose monitoring (blood glucose monitoring [BGM] group; n = 79).
Main outcomes and measures: The primary outcome was change in HbA1c from baseline to 26 weeks. There were 20 secondary outcomes, including additional HbA1c outcomes, CGM glucose metrics, and patient-reported outcomes with adjustment for multiple comparisons to control for the false discovery rate.
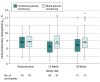
Results: Among the 153 participants (mean [SD] age, 17 [3] years; 76 [50%] were female; mean [SD] diabetes duration, 9 [5] years), 142 (93%) completed the study. In the CGM group, 68% of participants used CGM at least 5 days per week in month 6. Mean HbA1c was 8.9% at baseline and 8.5% at 26 weeks in the CGM group and 8.9% at both baseline and 26 weeks in the BGM group (adjusted between-group difference, -0.37% [95% CI, -0.66% to -0.08%]; P = .01). Of 20 prespecified secondary outcomes, there were statistically significant differences in 3 of 7 binary HbA1c outcomes, 8 of 9 CGM metrics, and 1 of 4 patient-reported outcomes. The most commonly reported adverse events in the CGM and BGM groups were severe hypoglycemia (3 participants with an event in the CGM group and 2 in the BGM group), hyperglycemia/ketosis (1 participant with an event in CGM group and 4 in the BGM group), and diabetic ketoacidosis (3 participants with an event in the CGM group and 1 in the BGM group).
Conclusions and relevance: Among adolescents and young adults with type 1 diabetes, continuous glucose monitoring compared with standard blood glucose monitoring resulted in a small but statistically significant improvement in glycemic control over 26 weeks. Further research is needed to understand the clinical importance of the findings.
Trial registration: ClinicalTrials.gov Identifier: NCT03263494.
Conflict of interest statement
Figures

Comment in
-
Continuous Glucose Monitoring in Adolescent, Young Adult, and Older Patients With Type 1 Diabetes.JAMA. 2020 Jun 16;323(23):2384-2385. doi: 10.1001/jama.2020.7058. JAMA. 2020. PMID: 32543670 Free PMC article. No abstract available.
References
-
- Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C; DIAMOND Study Group . Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND Trial. J Diabetes Sci Technol. 2017;11(6):1138-1146. doi: 10.1177/1932296817704445 - DOI - PMC - PubMed
-
- US Food and Drug Administration Premarket approval: Dexcom G5 Mobile Continuous Glucose Monitoring System. Updated May 11, 2020. Accessed May 15, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P120...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

