Effect of Ticagrelor Monotherapy vs Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome: The TICO Randomized Clinical Trial
- PMID: 32543684
- PMCID: PMC7298605
- DOI: 10.1001/jama.2020.7580
Effect of Ticagrelor Monotherapy vs Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome: The TICO Randomized Clinical Trial
Abstract
Importance: Discontinuing aspirin after short-term dual antiplatelet therapy (DAPT) was evaluated as a bleeding reduction strategy. However, the strategy of ticagrelor monotherapy has not been exclusively evaluated in patients with acute coronary syndromes (ACS).
Objective: To determine whether switching to ticagrelor monotherapy after 3 months of DAPT reduces net adverse clinical events compared with ticagrelor-based 12-month DAPT in patients with ACS treated with drug-eluting stents.
Design, setting, and participants: A randomized multicenter trial was conducted in 3056 patients with ACS treated with drug-eluting stents between August 2015 and October 2018 at 38 centers in South Korea. Follow-up was completed in October 2019.
Interventions: Patients were randomized to receive ticagrelor monotherapy (90 mg twice daily) after 3-month DAPT (n = 1527) or ticagrelor-based 12-month DAPT (n = 1529).
Main outcomes and measures: The primary outcome was a 1-year net adverse clinical event, defined as a composite of major bleeding and adverse cardiac and cerebrovascular events (death, myocardial infarction, stent thrombosis, stroke, or target-vessel revascularization). Prespecified secondary outcomes included major bleeding and major adverse cardiac and cerebrovascular events.
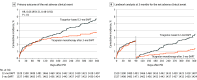
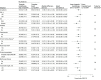
Results: Among 3056 patients who were randomized (mean age, 61 years; 628 women [20%]; 36% ST-elevation myocardial infarction), 2978 patients (97.4%) completed the trial. The primary outcome occurred in 59 patients (3.9%) receiving ticagrelor monotherapy after 3-month DAPT and in 89 patients (5.9%) receiving ticagrelor-based 12-month DAPT (absolute difference, -1.98% [95% CI, -3.50% to -0.45%]; hazard ratio [HR], 0.66 [95% CI, 0.48 to 0.92]; P = .01). Of 10 prespecified secondary outcomes, 8 showed no significant difference. Major bleeding occurred in 1.7% of patients with ticagrelor monotherapy after 3-month DAPT and in 3.0% of patients with ticagrelor-based 12-month DAPT (HR, 0.56 [95% CI, 0.34 to 0.91]; P = .02). The incidence of major adverse cardiac and cerebrovascular events was not significantly different between the ticagrelor monotherapy after 3-month DAPT group (2.3%) vs the ticagrelor-based 12-month DAPT group (3.4%) (HR, 0.69 [95% CI, 0.45 to 1.06]; P = .09).
Conclusions and relevance: Among patients with acute coronary syndromes treated with drug-eluting stents, ticagrelor monotherapy after 3 months of dual antiplatelet therapy, compared with ticagrelor-based 12-month dual antiplatelet therapy, resulted in a modest but statistically significant reduction in a composite outcome of major bleeding and cardiovascular events at 1 year. The study population and lower than expected event rates should be considered in interpreting the trial.
Trial registration: ClinicalTrials.gov Identifier: NCT02494895.
Conflict of interest statement
Figures

Comment in
-
In ACS treated with drug-eluting stents and 3 mo of DAPT, ticagrelor monotherapy reduced clinical events at 1 y vs. DAPT.Ann Intern Med. 2020 Oct 20;173(8):JC43. doi: 10.7326/ACPJ202010200-043. Ann Intern Med. 2020. PMID: 33075261
References
-
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115. doi: 10.1016/j.jacc.2016.03.513 - DOI - PubMed
-
- Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi: 10.1093/eurheartj/ehx419 - DOI - PubMed
-
- Park DW, Kwon O, Jang JS, et al. ; TICAKOREA Investigators . Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140(23):1865-1877. doi: 10.1161/CIRCULATIONAHA.119.041766 - DOI - PubMed

