The biogenesis and biology of amyloid β oligomers in the brain
- PMID: 32543725
- PMCID: PMC7984270
- DOI: 10.1002/alz.12084
The biogenesis and biology of amyloid β oligomers in the brain
Erratum in
-
Erratum.Alzheimers Dement. 2023 Jan 2. doi: 10.1002/alz.12791. Online ahead of print. Alzheimers Dement. 2023. PMID: 36591792 No abstract available.
Abstract
The repeated failure of clinical trials targeting the amyloid beta (Aβ) protein has challenged the amyloid cascade hypothesis. In this perspective, I discuss the biogenesis and biology of Aβ, from the arrangement of its atoms to its effects on the human brain. I hope that this analysis will help guide future attempts to home in on this elusive therapeutic target.
© 2020 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
No conflicts of interest
Figures
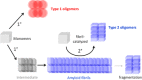
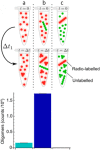
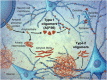

References
-
- Vassar R, Bennett BD, Babu‐Khan S, et al. Beta‐secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999; 286(5440): 735‐741. - PubMed
-
- De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of presenilin‐1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998; 391(6665): 387‐390. - PubMed
-
- Takasugi N, Tomita T, Hayashi I, et al. The role of presenilin cofactors in the gamma‐secretase complex. Nature. 2003; 422(6930): 438‐441. - PubMed
-
- Henley D, Raghavan N, Sperling R, Aisen P, Raman R, Romano G. Preliminary results of a trial of atabecestat in preclinical Alzheimer's disease. N Engl J Med. 2019; 380(15): 1483‐1485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

