Characterization of concurrent target suppression by JNJ-61178104, a bispecific antibody against human tumor necrosis factor and interleukin-17A
- PMID: 32544369
- PMCID: PMC7531573
- DOI: 10.1080/19420862.2020.1770018
Characterization of concurrent target suppression by JNJ-61178104, a bispecific antibody against human tumor necrosis factor and interleukin-17A
Abstract
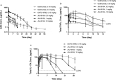
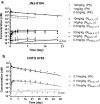
Tumor necrosis factor (TNF) and interleukin (IL)-17A are pleiotropic cytokines implicated in the pathogenesis of several autoimmune diseases including rheumatoid arthritis (RA) and psoriatic arthritis (PsA). JNJ-61178104 is a novel human anti-TNF and anti-IL-17A monovalent, bispecific antibody that binds to both human TNF and human IL-17A with high affinities and blocks the binding of TNF and IL-17A to their receptors in vitro. JNJ-61178104 also potently neutralizes TNF and IL-17A-mediated downstream effects in multiple cell-based assays. In vivo, treatment with JNJ-61178104 resulted in dose-dependent inhibition of cellular influx in a human IL-17A/TNF-induced murine lung neutrophilia model and the inhibitory effects of JNJ-61178104 were more potent than the treatment with bivalent parental anti-TNF or anti-IL-17A antibodies. JNJ-61178104 was shown to engage its targets, TNF and IL-17A, in systemic circulation measured as drug/target complex formation in normal cynomolgus monkeys (cyno). Surprisingly, quantitative target engagement assessment suggested lower apparent in vivo target-binding affinities for JNJ-61178104 compared to its bivalent parental antibodies, despite their similar in vitro target-binding affinities. The target engagement profiles of JNJ-61178104 in humans were in general agreement with the predicted profiles based on cyno data, suggesting similar differences in the apparent in vivo target-binding affinities. These findings show that in vivo target engagement of monovalent bispecific antibody does not necessarily recapitulate that of the molar-equivalent dose of its bivalent parental antibody. Our results also offer valuable insights into the understanding of the pharmacokinetics/pharmacodynamics and target engagement of other bispecific biologics against dimeric and/or trimeric soluble targets in vivo.
Keywords: IL-17A; TNF; bispecific antibody; target engagement; target-mediated drug disposition; translational modeling.
Figures






Similar articles
-
Pharmacokinetics, Pharmacodynamics, Immunogenicity, Safety, and Tolerability of JNJ-61178104, a Novel Tumor Necrosis Factor-Alpha and Interleukin-17A Bispecific Antibody, in Healthy Subjects.J Clin Pharmacol. 2019 Jul;59(7):968-978. doi: 10.1002/jcph.1393. Epub 2019 Feb 18. J Clin Pharmacol. 2019. PMID: 30776134 Clinical Trial.
-
Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases.MAbs. 2016;8(1):141-9. doi: 10.1080/19420862.2015.1093266. Epub 2015 Sep 22. MAbs. 2016. PMID: 26390837 Free PMC article. Clinical Trial.
-
Dual blockade of IL-17A and IL-36 pathways via a bispecific antibody exhibits enhanced anti-inflammatory potency.Front Immunol. 2024 Nov 12;15:1434127. doi: 10.3389/fimmu.2024.1434127. eCollection 2024. Front Immunol. 2024. PMID: 39600699 Free PMC article.
-
Secukinumab for rheumatology: development and its potential place in therapy.Drug Des Devel Ther. 2016 Jun 24;10:2069-80. doi: 10.2147/DDDT.S105263. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27445458 Free PMC article. Review.
-
Potential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumour necrosis factor and IL-1 in experimental arthritis.Ann Rheum Dis. 2006 Nov;65 Suppl 3(Suppl 3):iii29-33. doi: 10.1136/ard.2006.058529. Ann Rheum Dis. 2006. PMID: 17038468 Free PMC article. Review.
Cited by
-
The second decade of anti-TNF-a therapy in clinical practice: new lessons and future directions in the COVID-19 era.Rheumatol Int. 2022 Sep;42(9):1493-1511. doi: 10.1007/s00296-022-05136-x. Epub 2022 May 3. Rheumatol Int. 2022. PMID: 35503130 Free PMC article. Review.
-
Recent Advances in Translational Pharmacokinetics and Pharmacodynamics Prediction of Therapeutic Antibodies Using Modeling and Simulation.Pharmaceuticals (Basel). 2022 Apr 22;15(5):508. doi: 10.3390/ph15050508. Pharmaceuticals (Basel). 2022. PMID: 35631335 Free PMC article. Review.
-
The Role of Pharmacometrics in Advancing the Therapies for Autoimmune Diseases.Pharmaceutics. 2024 Dec 5;16(12):1559. doi: 10.3390/pharmaceutics16121559. Pharmaceutics. 2024. PMID: 39771538 Free PMC article. Review.
-
TRYBE®: an Fc-free antibody format with three monovalent targeting arms engineered for long in vivo half-life.MAbs. 2023 Jan-Dec;15(1):2160229. doi: 10.1080/19420862.2022.2160229. MAbs. 2023. PMID: 36788124 Free PMC article.
-
Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders.Signal Transduct Target Ther. 2024 Oct 4;9(1):263. doi: 10.1038/s41392-024-01952-8. Signal Transduct Target Ther. 2024. PMID: 39362875 Free PMC article. Review.
References
-
- Kotsovilis S, Andreakos E.. Therapeutic human monoclonal antibodies in inflammatory diseases. Methods Mol Biol. 2014;1060:37–14. - PubMed
-
- Fischer JA, Hueber AJ, Wilson S, Galm M, Baum W, Kitson C, Auer J, Lorenz SH, Moelleken J, Bader M, et al. Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 2015;67(1):51–62. doi:10.1002/art.38896. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
